Population Pharmacokinetic Analyses of Ertugliflozin in Select Ethnic Populations
- PMID: 34213819
- PMCID: PMC9291861
- DOI: 10.1002/cpdd.970
Population Pharmacokinetic Analyses of Ertugliflozin in Select Ethnic Populations
Abstract
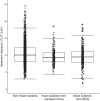
Ertugliflozin, a sodium-glucose cotransporter 2 inhibitor, is approved for treatment of type 2 diabetes. Two population pharmacokinetic (PK) analyses were conducted, using data from up to 17 phase 1 to 3 studies, to characterize ertugliflozin PK parameters in select ethnic subgroups: (1) East/Southeast (E/SE) Asian vs non-E/SE Asian subjects; (2) Asian subjects from mainland China vs Asian subjects from the rest of the world and non-Asian subjects. A 2-compartment model with first-order absorption, lag time, and first-order elimination was fitted to the observed data. For the E/SE Asian vs non-E/SE Asian analysis (13 692 PK observations from 2276 subjects), E/SE Asian subjects exhibited a 17% increase in apparent clearance (CL/F) and 148% increase in apparent central volume of distribution (Vc/F) vs non-E/SE Asian subjects. However, individual post hoc CL/F values were similar between groups when body weight differences were considered. For the second analysis (16 018 PK observations from 2620 subjects), compared with non-Asian subjects, CL/F was similar while Vc/F increased by 44% in Asian subjects from mainland China and both CL/F and Vc/F increased in Asian subjects from the rest of the world (8% and 115%, respectively) vs non-Asian subjects. Increases in Vc/F would decrease the ertugliflozin maximum concentration but would not impact area under the concentration-time curve. Therefore, the differences in CL/F (area under the concentration-time curve) and Vc/F were not considered clinically relevant or likely to result in meaningful ethnic differences in the PK of ertugliflozin.
Trial registration: ClinicalTrials.gov NCT00989079 NCT01127308 NCT01054300 NCT01223339 NCT01948986 NCT01096667 NCT01059825 NCT01986855 NCT02033889 NCT02099110 NCT01958671 NCT02630706.
Keywords: diabetes; ertugliflozin; population pharmacokinetics; sodium-glucose cotransporter 2 inhibitor.
© 2021 Merck Sharp & Dohme Corp and Pifzer Inc. Clinical Pharmacology in Drug Development published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
D.J.F., V.S., V.K.D., and K.S. are employees of Pfizer Inc., New York, NY, USA and may own shares/stock options in Pfizer Inc. S.Z. is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may own stock in Merck & Co., Inc., Kenilworth, NJ, USA.
Figures

References
-
- International Diabetes Federation . IDF Diabetes Atlas, 9th ed. Brussels, Belgium. https://www.diabetesatlas.org. Published 2019. Accessed December 18, 2020.
-
- Kalgutkar AS, Tugnait M, Zhu T, et al. Preclinical species and human disposition of PF‐04971729, a selective inhibitor of the sodium‐dependent glucose cotransporter 2 and clinical candidate for the treatment of type 2 diabetes mellitus. Drug Metab Dispos. 2011;39(9):1609‐1619. - PubMed
-
- Miao Z, Nucci G, Amin N, et al. Pharmacokinetics, metabolism, and excretion of the antidiabetic agent ertugliflozin (PF‐04971729) in healthy male subjects. Drug Metab Dispos. 2013;41(2):445‐456. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

