Pathogenic Variants Associated With Dilated Cardiomyopathy Predict Outcome in Pediatric Myocarditis
- PMID: 34213952
- PMCID: PMC8373449
- DOI: 10.1161/CIRCGEN.120.003250
Pathogenic Variants Associated With Dilated Cardiomyopathy Predict Outcome in Pediatric Myocarditis
Abstract
Background: Myocarditis is one of the most common causes leading to heart failure in children and a possible genetic background has been postulated. We sought to characterize the clinical and genetic characteristics in patients with myocarditis ≤18 years of age to predict outcome.
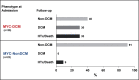
Methods: A cohort of 42 patients (Genetics in Pediatric Myocarditis) with biopsy-proven myocarditis underwent genetic testing with targeted panel sequencing of cardiomyopathy-associated genes. Genetics in Pediatric Myocarditis patients were divided into subgroups according to the phenotype of dilated cardiomyopathy (DCM) at presentation, resulting in 22 patients without DCM (myocarditis without phenotype of DCM) and 20 patients with DCM (myocarditis with phenotype of DCM).
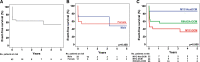
Results: Myocarditis with phenotype of DCM patients (median age 1.4 years) were younger than myocarditis without phenotype of DCM patients (median age 16.1 years; P<0.001) and were corresponding to heart failure-like and coronary syndrome-like phenotypes, respectively. At least one likely pathogenic/pathogenic variant was identified in 9 out of 42 patients (22%), 8 of them were heterozygous, and 7 out of 9 were in myocarditis with phenotype of DCM. Likely pathogenic/pathogenic variants were found in genes validated for primary DCM (BAG3, DSP, LMNA, MYH7, TNNI3, TNNT2, and TTN). Rare variant enrichment analysis revealed significant accumulation of high-impact disease variants in myocarditis with phenotype of DCM versus healthy individuals (P=0.0003). Event-free survival was lower (P=0.008) in myocarditis with phenotype of DCM patients compared with myocarditis without phenotype of DCM and primary DCM.
Conclusions: We report heterozygous likely pathogenic/pathogenic variants in biopsy-proven pediatric myocarditis. Myocarditis patients with DCM phenotype were characterized by early-onset heart failure, significant enrichment of likely pathogenic/pathogenic variants, and poor outcome. These phenotype-specific and age group-specific findings will be useful for personalized management of these patients. Genetic evaluation in children newly diagnosed with myocarditis and DCM phenotype is warranted.
Keywords: biopsy, endomyocardial; cardiomyopathy, dilated; genetics; myocarditis.
Figures


References
-
- Rossano JW, Shaddy RE. Heart failure in children: etiology and treatment. J Pediatr. 2014;165:228–233. doi: 10.1016/j.jpeds.2014.04.055 - PubMed
-
- Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, et al. . Report of the 1995 world health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841 - PubMed
-
- Canter CE, Simpson KE, Simpson KP. Diagnosis and treatment of myocarditis in children in the current era. Circulation. 2014;129:115–128. doi: 10.1161/CIRCULATIONAHA.113.001372 - PubMed
-
- Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, et al. ; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–48, 2648a. doi: 10.1093/eurheartj/eht210 - PubMed
-
- Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, et al. . Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

