Chimeric spike mRNA vaccines protect against Sarbecovirus challenge in mice
- PMID: 34214046
- PMCID: PMC8899822
- DOI: 10.1126/science.abi4506
Chimeric spike mRNA vaccines protect against Sarbecovirus challenge in mice
Abstract
The emergence of severe acute respiratory syndrome coronavirus (SARS-CoV) in 2003 and SARS-CoV-2 in 2019 highlights the need to develop universal vaccination strategies against the broader Sarbecovirus subgenus. Using chimeric spike designs, we demonstrate protection against challenge from SARS-CoV, SARS-CoV-2, SARS-CoV-2 B.1.351, bat CoV (Bt-CoV) RsSHC014, and a heterologous Bt-CoV WIV-1 in vulnerable aged mice. Chimeric spike messenger RNAs (mRNAs) induced high levels of broadly protective neutralizing antibodies against high-risk Sarbecoviruses. By contrast, SARS-CoV-2 mRNA vaccination not only showed a marked reduction in neutralizing titers against heterologous Sarbecoviruses, but SARS-CoV and WIV-1 challenge in mice resulted in breakthrough infections. Chimeric spike mRNA vaccines efficiently neutralized D614G, mink cluster five, and the UK B.1.1.7 and South African B.1.351 variants of concern. Thus, multiplexed-chimeric spikes can prevent SARS-like zoonotic coronavirus infections with pandemic potential.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures

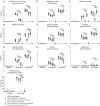
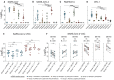



Update of
-
Chimeric spike mRNA vaccines protect against Sarbecovirus challenge in mice.bioRxiv [Preprint]. 2021 May 11:2021.03.11.434872. doi: 10.1101/2021.03.11.434872. bioRxiv. 2021. Update in: Science. 2021 Aug 27;373(6558):991-998. doi: 10.1126/science.abi4506. PMID: 33758837 Free PMC article. Updated. Preprint.
References
-
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L., A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). 10.1038/s41586-020-2012-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 AI152296/AI/NIAID NIH HHS/United States
- R01 AI110700/AI/NIAID NIH HHS/United States
- T32 AI007151/AI/NIAID NIH HHS/United States
- R01 AI157155/AI/NIAID NIH HHS/United States
- R01 AI108197/AI/NIAID NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- U54 CA260543/CA/NCI NIH HHS/United States
- R01 AI124429/AI/NIAID NIH HHS/United States
- U01 AI149644/AI/NIAID NIH HHS/United States
- U19 AI142759/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 AI132178/AI/NIAID NIH HHS/United States
- U19 AI100625/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

