Selective Bond Cleavage in RAFT Agents Promoted by Low-Energy Electron Attachment
- PMID: 34214239
- PMCID: PMC8456798
- DOI: 10.1002/anie.202107480
Selective Bond Cleavage in RAFT Agents Promoted by Low-Energy Electron Attachment
Abstract
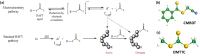
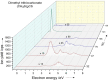
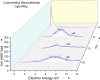
Radical polymerization with reversible addition-fragmentation chain transfer (RAFT polymerization) has been successfully applied to generate polymers of well-defined architecture. For RAFT polymerization a source of radicals is required. Recent work has demonstrated that for minimal side-reactions and high spatio-temporal control these should be formed directly from the RAFT agent or macroRAFT agent (usually carbonothiosulfanyl compounds) thermally, photochemically or by electrochemical reduction. In this work, we investigated low-energy electron attachment to a common RAFT agent (cyanomethyl benzodithioate), and, for comparison, a simple carbonothioylsulfanyl compound (dimethyl trithiocarbonate, DMTTC) in the gas phase by means of mass spectrometry as well as quantum chemical calculations. We observe for both compounds that specific cleavage of the C-S bond is induced upon low-energy electron attachment at electron energies close to zero eV. This applies even in the case of a poor homolytic leaving group (. CH3 in DMTTC). All other dissociation reactions found at higher electron energies are much less abundant. The present results show a high control of the chemical reactions induced by electron attachment.
Keywords: RAFT agents; dimethyl trithiocarbonate; dissociative electron attachment; low-energy electron; radical polymerization.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Corrigan N., Jung K., Moad G., Hawker C. J., Matyjaszewski K., Boyer C., Prog. Polym. Sci. 2020, 111, 101311.
-
- Braunecker W. A., Matyjaszewski K., Prog. Polym. Sci. 2007, 32, 93–146.
-
- Goto A., Fukuda T., Prog. Polym. Sci. 2004, 29, 329–385.
-
- Wang J. S., Matyjaszewski K., J. Am. Chem. Soc. 1995, 117, 5614–5615.
-
- Matyjaszewski K., Xia J. H., Chem. Rev. 2001, 101, 2921–2990. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

