Fundamental limits on the rate of bacterial growth and their influence on proteomic composition
- PMID: 34214468
- PMCID: PMC8460600
- DOI: 10.1016/j.cels.2021.06.002
Fundamental limits on the rate of bacterial growth and their influence on proteomic composition
Abstract
Despite abundant measurements of bacterial growth rate, cell size, and protein content, we lack a rigorous understanding of what sets the scale of these quantities and when protein abundances should (or should not) depend on growth rate. Here, we estimate the basic requirements and physical constraints on steady-state growth by considering key processes in cellular physiology across a collection of Escherichia coli proteomic data covering ≈4,000 proteins and 36 growth rates. Our analysis suggests that cells are predominantly tuned for the task of cell doubling across a continuum of growth rates; specific processes do not limit growth rate or dictate cell size. We present a model of proteomic regulation as a function of nutrient supply that reconciles observed interdependences between protein synthesis, cell size, and growth rate and propose that a theoretical inability to parallelize ribosomal synthesis places a firm limit on the achievable growth rate. A record of this paper's transparent peer review process is included in the supplemental information.
Keywords: bacteria; cell size; cellular growth; microbial growth laws; microbial physiology; order-of-magnitude estimation; physical biology; proteomics.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.A.T. is chief scientific advisor at the Allen Institute for Cell Science (Seattle, WA, 98109). The authors otherwise declare no competing interests.
Figures



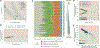

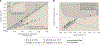

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

