Photoreceptor Cell Calcium Dysregulation and Calpain Activation Promote Pathogenic Photoreceptor Oxidative Stress and Inflammation in Prodromal Diabetic Retinopathy
- PMID: 34214506
- PMCID: PMC8579242
- DOI: 10.1016/j.ajpath.2021.06.006
Photoreceptor Cell Calcium Dysregulation and Calpain Activation Promote Pathogenic Photoreceptor Oxidative Stress and Inflammation in Prodromal Diabetic Retinopathy
Abstract
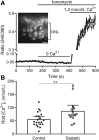
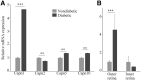
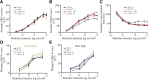
This study tested the hypothesis that diabetes promotes a greater than normal cytosolic calcium level in rod cells that activates a Ca2+-sensitive protease, calpain, resulting in oxidative stress and inflammation, two pathogenic factors of early diabetic retinopathy. Nondiabetic and 2-month diabetic C57Bl/6J and calpain1 knockout (Capn1-/-) mice were studied; subgroups were treated with a calpain inhibitor (CI). Ca2+ content was measured in photoreceptors using Fura-2. Retinal calpain expression was studied by quantitative RT-PCR and immunohistochemistry. Superoxide and expression of inflammatory proteins were measured using published methods. Proteomic analysis was conducted on photoreceptors isolated from untreated diabetic mice or treated daily with CI for 2 months. Cytosolic Ca2+ content was increased twofold in photoreceptors of diabetic mice as compared with nondiabetic mice. Capn1 expression increased fivefold in photoreceptor outer segments of diabetic mice. Pharmacologic inhibition or genetic deletion of Capn1 significantly suppressed diabetes-induced oxidative stress and expression of proinflammatory proteins in retina. Proteomics identified a protein (WW domain-containing oxidoreductase [WWOX]) whose expression was significantly increased in photoreceptors from mice diabetic for 2 months and was inhibited with CI. Knockdown of Wwox using specific siRNA in vitro inhibited increase in superoxide caused by the high glucose. These results suggest that reducing Ca2+ accumulation, suppressing calpain activation, and/or reducing Wwox up-regulation are novel targets for treating early diabetic retinopathy.
Copyright © 2021 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

