Three-month analysis of total humoral response to Pfizer BNT162b2 mRNA COVID-19 vaccination in healthcare workers
- PMID: 34214516
- PMCID: PMC8241575
- DOI: 10.1016/j.jinf.2021.06.024
Three-month analysis of total humoral response to Pfizer BNT162b2 mRNA COVID-19 vaccination in healthcare workers
Keywords: Antibodies; COVID-19; Immune response; SARS-CoV-2; Vaccination.
Conflict of interest statement
Declaration of Competing Interest The authors have no relevant competing interest to disclose in relation to this work.
Figures
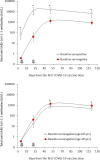
Comment on
-
Waning antibodies in SARS-CoV-2 naïve vaccinees: Results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers.J Infect. 2021 Sep;83(3):381-412. doi: 10.1016/j.jinf.2021.06.017. Epub 2021 Jun 20. J Infect. 2021. PMID: 34161817 Free PMC article. No abstract available.
References
-
- Tré-Hardy M., Cupaiolo R., Wilmet A., Beukinga I., Blairon L. Waning antibodies in SARS-CoV-2 naïve vaccinees: results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers. J Infect. 2021 doi: 10.1016/j.jinf.2021.06.017. Jun 20:S0163-4453(21)00314-5Epub ahead of print. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

