A tridomain model for potassium clearance in optic nerve of Necturus
- PMID: 34214534
- PMCID: PMC8390971
- DOI: 10.1016/j.bpj.2021.06.020
A tridomain model for potassium clearance in optic nerve of Necturus
Abstract
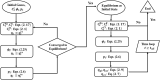
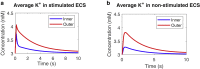
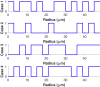
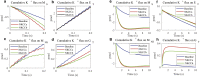
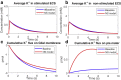
Complex fluids flow in complex ways in complex structures. Transport of water and various organic and inorganic molecules in the central nervous system are important in a wide range of biological and medical processes. However, the exact driving mechanisms are often not known. In this work, we investigate flows induced by action potentials in an optic nerve as a prototype of the central nervous system. Different from traditional fluid dynamics problems, flows in biological tissues such as the central nervous system are coupled with ion transport. They are driven by osmosis created by concentration gradient of ionic solutions, which in turn influence the transport of ions. Our mathematical model is based on the known structural and biophysical properties of the experimental system used by the Harvard group Orkand et al. Asymptotic analysis and numerical computation show the significant role of water in convective ion transport. The full model (including water) and the electrodiffusion model (excluding water) are compared in detail to reveal an interesting interplay between water and ion transport. In the full model, convection due to water flow dominates inside the glial domain. This water flow in the glia contributes significantly to the spatial buffering of potassium in the extracellular space. Convection in the extracellular domain does not contribute significantly to spatial buffering. Electrodiffusion is the dominant mechanism for flows confined to the extracellular domain.
Copyright © 2021 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
















References
-
- Hodgkin A.L., Huxley A.F., Katz B. Ionic currents underlying activity in the giant axon of the squid. Arch. Sci. Physiol. (Paris) 1949;3:129–150.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

