Structural perspectives on H2S homeostasis
- PMID: 34214926
- PMCID: PMC8648995
- DOI: 10.1016/j.sbi.2021.05.010
Structural perspectives on H2S homeostasis
Abstract
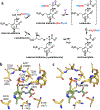
The enzymes involved in H2S homeostasis regulate its production from sulfur-containing amino acids and its oxidation to thiosulfate and sulfate. Two gatekeepers in this homeostatic circuit are cystathionine beta-synthase, which commits homocysteine to cysteine, and sulfide quinone oxidoreductase, which commits H2S to oxidation via a mitochondrial pathway. Inborn errors at either locus affect sulfur metabolism, increasing homocysteine-derived H2S synthesis in the case of CBS deficiency and reducing complex IV activity in the case of SQOR deficiency. In this review, we focus on structural perspectives on the reaction mechanisms and regulation of these two enzymes, which are key to understanding H2S homeostasis in health and its dysregulation and potential targeting in disease.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement R.B. is a paid member of the scientific advisory board of Apneo Therapeutics and owns equity in the company.
Figures
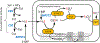

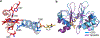
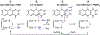

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

