Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial
- PMID: 34215210
- PMCID: PMC8250562
- DOI: 10.1186/s12879-021-06348-5
Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has changed our lives. The scientific community has been investigating re-purposed treatments to prevent disease progression in coronavirus disease (COVID-19) patients.
Objective: To determine whether ivermectin treatment can prevent hospitalization in individuals with early COVID-19.
Design, setting and participants: A randomized, double-blind, placebo-controlled study was conducted in non-hospitalized individuals with COVID-19 in Corrientes, Argentina. Patients with SARS-CoV-2 positive nasal swabs were contacted within 48 h by telephone to invite them to participate. The trial randomized 501 patients between August 19th 2020 and February 22nd 2021.
Intervention: Patients were randomized to ivermectin (N = 250) or placebo (N = 251) arms in a staggered dose, according to the patient's weight, for 2 days.
Main outcomes and measures: The efficacy of ivermectin to prevent hospitalizations was evaluated as primary outcome. We evaluated secondary outcomes in relationship to safety and other efficacy end points.
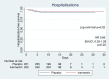
Results: The mean age was 42 years (SD ± 15.5) and the median time since symptom onset to the inclusion was 4 days [interquartile range 3-6]. The primary outcome of hospitalization was met in 14/250 (5.6%) individuals in ivermectin group and 21/251 (8.4%) in placebo group (odds ratio 0.65; 95% confidence interval, 0.32-1.31; p = 0.227). Time to hospitalization was not statistically different between groups. The mean time from study enrollment to invasive mechanical ventilatory support (MVS) was 5.25 days (SD ± 1.71) in ivermectin group and 10 days (SD ± 2) in placebo group, (p = 0.019). There were no statistically significant differences in the other secondary outcomes including polymerase chain reaction test negativity and safety outcomes.
Limitations: Low percentage of hospitalization events, dose of ivermectin and not including only high-risk population.
Conclusion: Ivermectin had no significant effect on preventing hospitalization of patients with COVID-19. Patients who received ivermectin required invasive MVS earlier in their treatment. No significant differences were observed in any of the other secondary outcomes.
Trial registration: ClinicalTrials.gov NCT04529525 .
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Johns Hopkins University & Medicine. Coronavirus resource center. Accessed 22 Mar 2021. https://coronavirus.jhu.edu.
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, Ko WC, Hsueh PR. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous