Comparative cytokine profiling identifies common and unique serum cytokine responses in acute chikungunya and dengue virus infection
- PMID: 34215212
- PMCID: PMC8254284
- DOI: 10.1186/s12879-021-06339-6
Comparative cytokine profiling identifies common and unique serum cytokine responses in acute chikungunya and dengue virus infection
Abstract
Background: Infection by chikungunya (CHIKV) and dengue virus (DENV) can cause a wide spectrum of clinical features, many of which are undifferentiated. Cytokines, which broadly also include chemokines and growth factors, have been shown to play a role in protective immunity as well as DENV and CHIKV pathogenesis. However, differences in cytokine response to both viruses remain poorly understood, especially in patients from countries where both viruses are endemic. Our study is therefore aimed to provide a comparative profiling of cytokine response induced by acute DENV and CHIKV infections in patients with similar disease stages and in experimental in vitro infections.
Methods: By using multiplex immunoassay, we compared host cytokine profiles between acute CHIKV and DENV infections by analysing serum cytokine levels of IL-1α, IL-4, IL-5, IL-8, IL-13, RANTES, MCP-3, eotaxin, PDGF-AB/BB, and FGF-2 from the sera of acute chikungunya and dengue fever patients. We further investigated the cytokine profile responses using experimental in vitro CHIKV and DENV infections of peripheral blood mononuclear cells (PBMCs).
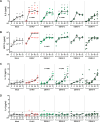
Results: We found that both CHIKV and DENV-infected patients had an upregulated level of IL-8 and IL-4, with the highest IL-4 level observed in DENV-2 infected patients. Higher IL-8 level was also correlated with lower platelet count in dengue patients. IL-13 and MCP-3 downregulation was observed only in chikungunya patients, while conversely PDGF-AB/BB and FGF-2 downregulation was unique in dengue patients. Age-associated differential expression of IL-13, MCP-3, and IL-5 was also observed, while distinct kinetics of IL-4, IL-8, and FGF-2 expression between CHIKV and DENV-infected patients were identified. Furthermore, the unique pattern of IL-8, IL-13 and MCP-3, but not IL-4 expression was also recapitulated using experimental in vitro infection in PBMCs.
Conclusions: Taken together, our study identified common cytokine response profile characterized by upregulation of IL-8 and IL-4 between CHIKV and DENV infection. Downregulation of IL-13 and MCP-3 was identified as a unique cytokine response profile of acute CHIKV infection, while distinct downregulation of PDGF-AB/BB and FGF-2 characterized the response from acute DENV infection. Our study provides an important overview of the host cytokine responses between CHIKV and DENV infection, which is important to further understand the mechanism and pathology of these diseases.
Keywords: Chikungunya; Cytokine; Dengue; Host response.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, Hay SI, Bedi N, Bensenor IM, Castañeda-Orjuela CA, Chuang TW, Gibney KB, Memish ZA, Rafay A, Ukwaja KN, Yonemoto N, Murray CJL. The global burden of dengue: an analysis from the global burden of disease study 2013. Lancet Infect Dis. 2016;16(6):712–723. doi: 10.1016/S1473-3099(16)00026-8. - DOI - PMC - PubMed
-
- Shahid U, Farooqi JQ, Barr KL, Mahmoodid SF, Jamil B, Imitaz K, et al. Comparison of clinical presentation and out-comes of Chikungunya and dengue virus infections in patients with acute undifferentiated febrile illness from the Sindh region of Pakistan. PLoS Negl Trop Dis. 2020;14(3):1–13. doi: 10.1371/journal.pntd.0008086. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

