Improvements in clinical signs of Parkinson's disease using photobiomodulation: a prospective proof-of-concept study
- PMID: 34215216
- PMCID: PMC8249215
- DOI: 10.1186/s12883-021-02248-y
Improvements in clinical signs of Parkinson's disease using photobiomodulation: a prospective proof-of-concept study
Abstract
Background: Parkinson's disease (PD) is a progressive neurodegenerative disease with no cure and few treatment options. Its incidence is increasing due to aging populations, longer disease duration and potentially as a COVID-19 sequela. Photobiomodulation (PBM) has been successfully used in animal models to reduce the signs of PD and to protect dopaminergic neurons.
Objective: To assess the effectiveness of PBM to mitigate clinical signs of PD in a prospective proof-of-concept study, using a combination of transcranial and remote treatment, in order to inform on best practice for a larger randomized placebo-controlled trial (RCT).
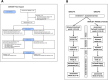
Methods: Twelve participants with idiopathic PD were recruited. Six were randomly chosen to begin 12 weeks of transcranial, intranasal, neck and abdominal PBM. The remaining 6 were waitlisted for 14 weeks before commencing the same treatment. After the 12-week treatment period, all participants were supplied with PBM devices to continue home treatment. Participants were assessed for mobility, fine motor skills, balance and cognition before treatment began, after 4 weeks of treatment, after 12 weeks of treatment and the end of the home treatment period. A Wilcoxon Signed Ranks test was used to assess treatment effectiveness at a significance level of 5%.
Results: Measures of mobility, cognition, dynamic balance and fine motor skill were significantly improved (p < 0.05) with PBM treatment for 12 weeks and up to one year. Many individual improvements were above the minimal clinically important difference, the threshold judged to be meaningful for participants. Individual improvements varied but many continued for up to one year with sustained home treatment. There was a demonstrable Hawthorne Effect that was below the treatment effect. No side effects of the treatment were observed.
Conclusions: PBM was shown to be a safe and potentially effective treatment for a range of clinical signs and symptoms of PD. Improvements were maintained for as long as treatment continued, for up to one year in a neurodegenerative disease where decline is typically expected. Home treatment of PD by the person themselves or with the help of a carer might be an effective therapy option. The results of this study indicate that a large RCT is warranted.
Trial registration: Australian New Zealand Clinical Trials Registry, registration number: ACTRN12618000038291p , registered on 12/01/2018.
Keywords: Cognition; Mobility; Motor symptoms; Parkinson’s disease; Photobiomodulation.
Conflict of interest statement
AL and BB, since February 2020, are co-founders, directors and current employees of SYMBYX Pty Ltd., a med-tech company developing treatments for neurological disorders. BB is an agent for Spectro Analytic Irradia AB, a laser manufacturer. The other authors declare that they have no competing interests.
Figures


References
-
- Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C, on behalf of the Movement Disorder Society Evidence-Based Medicine Committee International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson's disease. Mov Disord. 2018;33(8):1248–1266. doi: 10.1002/mds.27372. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

