CD36 promotes vasculogenic mimicry in melanoma by mediating adhesion to the extracellular matrix
- PMID: 34215227
- PMCID: PMC8254274
- DOI: 10.1186/s12885-021-08482-4
CD36 promotes vasculogenic mimicry in melanoma by mediating adhesion to the extracellular matrix
Abstract
Background: The formation of blood vessels within solid tumors directly contributes to cancer growth and metastasis. Until recently, tumor vasculature was thought to occur exclusively via endothelial cell (EC) lined structures (i.e. angiogenesis), but a second source of tumor vasculature arises from the cancer cells themselves, a process known as vasculogenic mimicry (VM). While it is generally understood that the function of VM vessels is the same as that of EC-lined vessels (i.e. to supply oxygen and nutrients to the proliferating cancer cells), the molecular mechanisms underpinning VM are yet to be fully elucidated.
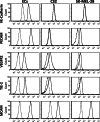
Methods: Human VM-competent melanoma cell lines were examined for their VM potential using the in vitro angiogenesis assays (Matrigel), together with inhibition studies using small interfering RNA and blocking monoclonal antibodies. Invasion assays and adhesion assays were used to examine cancer cell function.
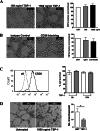
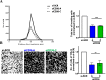
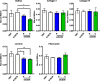
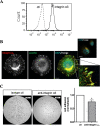
Results: Herein we demonstrate that CD36, a cell surface glycoprotein known to promote angiogenesis by ECs, also supports VM formation by human melanoma cancer cells. In silico analysis of CD36 expression within the melanoma cohort of The Cancer Genome Atlas suggests that melanoma patients with high expression of CD36 have a poorer clinical outcome. Using in vitro 'angiogenesis' assays and CD36-knockdown approaches, we reveal that CD36 supports VM formation by human melanoma cells as well as adhesion to, and invasion through, a cancer derived extracellular matrix substrate. Interestingly, thrombospondin-1 (TSP-1), a ligand for CD36 on ECs that inhibits angiogenesis, has no effect on VM formation. Further investigation revealed a role for laminin, but not collagen or fibronectin, as ligands for CD36 expressing melanoma cells.
Conclusions: Taken together, this study suggests that CD36 is a novel regulator of VM by melanoma cancer cells that is facilitated, at least in part, via integrin-α3 and laminin. Unlike angiogenesis, VM is not perturbed by the presence of TSP-1, thus providing new information on differences between these two processes of tumor vascularization which may be exploited to combat cancer progression.
Keywords: CD36; Integrin; Laminin; Melanoma; Thrombospondin; Tumor microenvironment; Vasculogenic mimicry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

