Making patient blood management the new norm(al) as experienced by implementors in diverse countries
- PMID: 34215251
- PMCID: PMC8249439
- DOI: 10.1186/s12913-021-06484-3
Making patient blood management the new norm(al) as experienced by implementors in diverse countries
Abstract
Background: Patient blood management (PBM) describes a set of evidence-based practices to optimize medical and surgical patient outcomes by clinically managing and preserving a patient's own blood. This concepts aims to detect and treat anemia, minimize the risk for blood loss and the need for blood replacement for each patient through a coordinated multidisciplinary care process. In combination with blood loss, anemia is the main driver for transfusion and all three are independent risk factors for adverse outcomes including morbidity and mortality. Evidence demonstrates that PBM significantly improves outcomes and safety while reducing cost by macroeconomic magnitudes. Despite its huge potential to improve healthcare systems, PBM is not yet adopted broadly. The aim of this study is to analyze the collective experiences of a diverse group of PBM implementors across countries reflecting different healthcare contexts and to use these experiences to develop a guidance for initiating and orchestrating PBM implementation for stakeholders from diverse professional backgrounds.
Methods: Semi-structured interviews were conducted with 1-4 PBM implementors from 12 countries in Asia, Latin America, Australia, Central and Eastern Europe, the Middle East, and Africa. Responses reflecting the drivers, barriers, measures, and stakeholders regarding the implementation of PBM were summarized per country and underwent qualitative content analysis. Clustering the resulting implementation measures by levels of intervention for PBM implementation informed a PBM implementation framework.
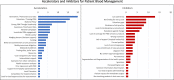
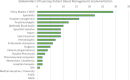
Results: A set of PBM implementation measures were extracted from the interviews with the implementors. Most of these measures relate to one of six levels of implementation including government, healthcare providers, funding, research, training/education, and patients/public. Essential cross-level measures are multi-stakeholder communication and collaboration.
Conclusion: The implementation matrix resulting from this research helps to decompose the complexity of PBM implementation into concrete measures on each implementation level. It provides guidance for diverse stakeholders to design, initiate and develop strategies and plans to make PBM a national standard of care, thus closing current practice gaps and matching this unmet public health need.
Keywords: Culture change; Implementation; Patient blood management; Patient outcomes; Practice change; Transfusion.
Conflict of interest statement
AH received honoraria and/or travel support from Celgene (Belgium), Instrumentation Laboratories/Werfen (USA), G1 Therapeutics (USA), the South African National Blood Service and Vifor Pharma, Switzerland.
DRS’s academic department is receiving grant support from the Swiss National Science Foundation, Berne, Switzerland, the Swiss Society of Anesthesiology and Reanimation (SGAR), Berne, Switzerland, the Swiss Foundation for Anesthesia Research, Zurich, Switzerland, Vifor SA, Villars-sur-Glâne, Switzerland. DRS is co-chair of the ABC-Trauma Faculty, sponsored by unrestricted educational grants from Novo Nordisk Health Care AG, Zurich, Switzerland, CSL Behring GmbH, Marburg, Germany, LFB Biomédicaments, Courtaboeuf Cedex, France and Octapharma AG, Lachen, Switzerland. DRS received honoraria / travel support for consulting or lecturing from: Danube University of Krems, Austria, US Department of Defense, Washington, USA, European Society of Anesthesiology, Brussels, BE, Korean Society for Patient Blood Management, Seoul, Korea, Korean Society of Anesthesiologists, Seoul, Korea, Network for the Advancement of Patient Blood Management, Haemostasis and Thrombosis, Paris, France, Baxalta Switzerland AG, Volketswil, Switzerland, Bayer AG, Zürich, Switzerland, B. Braun Melsungen AG, Melsungen, Germany, Boehringer Ingelheim GmbH, Basel, Switzerland, Bristol-Myers-Squibb, Rueil-Malmaison Cedex, France and Baar, Switzerland, CSL Behring GmbH, Hattersheim am Main, Germany and Berne, Switzerland, Celgene International II Sàrl, Couvet, Switzerland, Daiichi Sankyo AG, Thalwil, Switzerland, Ethicon Sàrl, Neuchâtel, Switzerland, Haemonetics, Braintree, MA, USA, Instrumentation Laboratory (Werfen), Bedford, MA, USA, LFB Biomédicaments, Courtaboeuf Cedex, France, Merck Sharp & Dohme, Kenilworth, New Jersey, USA, PAION Deutschland GmbH, Aachen, Germany, Pharmacosmos A/S, Holbaek, Denmark, Photonics Healthcare B.V., Utrecht, Netherlands, Pfizer AG, Zürich, Switzerland, Pierre Fabre Pharma, Alschwil, Switzerland, Roche Diagnostics International Ltd., Reinach, Switzerland, Sarstedt AG & Co., Sevelen, Switzerland and Nümbrecht, Germany, Shire Switzerland GmbH, Zug, Switzerland, Tem International GmbH, Munich, Germany, Vifor Pharma, Munich, Germany, Neuilly sur Seine, France and Villars-sur-Glâne, Switzerland, Vifor (International) AG, St. Gallen, Switzerland, Zuellig Pharma Holdings, Singapore, Singapore.
APH is employed by Health Outcomes Strategies GmbH (Switzerland) and has consulted and advised several pharmaceutical companies including Vifor Pharma AG, Novartis, and Abbott Laboratories. Health Outcomes Strategies GmbH has received funding for this research and for writing the manuscript.
Figures


References
-
- Muñoz M, Gómez-Ramírez S, Kozek-Langeneker S, Shander A, Richards T, Pavía J, Kehlet H, Acheson AG, Evans C, Raobaikady R, Javidroozi M, Auerbach M. “Fit to fly”: overcoming barriers to preoperative haemoglobin optimization in surgical patients. Br J Anaesth. 2015;115(1):15–24. doi: 10.1093/bja/aev165. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

