Adhesion dynamics in the neocortex determine the start of migration and the post-migratory orientation of neurons
- PMID: 34215578
- PMCID: PMC11060048
- DOI: 10.1126/sciadv.abf1973
Adhesion dynamics in the neocortex determine the start of migration and the post-migratory orientation of neurons
Abstract
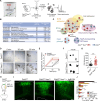
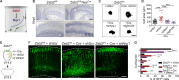
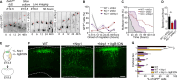
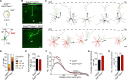
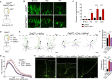
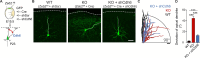
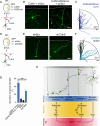
The neocortex is stereotypically organized into layers of excitatory neurons arranged in a precise parallel orientation. Here we show that dynamic adhesion both preceding and following radial migration is essential for this organization. Neuronal adhesion is regulated by the Mowat-Wilson syndrome-associated transcription factor Zeb2 (Sip1/Zfhx1b) through direct repression of independent adhesion pathways controlled by Neuropilin-1 (Nrp1) and Cadherin-6 (Cdh6). We reveal that to initiate radial migration, neurons must first suppress adhesion to the extracellular matrix. Zeb2 regulates the multipolar stage by transcriptional repression of Nrp1 and thereby downstream inhibition of integrin signaling. Upon completion of migration, neurons undergo an orientation process that is independent of migration. The parallel organization of neurons within the neocortex is controlled by Cdh6 through atypical regulation of integrin signaling via its RGD motif. Our data shed light on the mechanisms that regulate initiation of radial migration and the postmigratory orientation of neurons during neocortical development.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Schuster S., Rivalan M., Strauss U., Stoenica L., Trimbuch T., Rademacher N., Parthasarathy S., Lajkó D., Rosenmund C., Shoichet S. A., Winter Y., Tarabykin V., Rosário M., NOMA-GAP/ARHGAP33 regulates synapse development and autistic-like behavior in the mouse. Mol. Psychiatry 20, 1120–1131 (2015). - PubMed
-
- Kaufmann W. E., Moser H. W., Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex 10, 981–991 (2000). - PubMed
-
- Nadarajah B., Brunstrom J. E., Grutzendler J., Wong R. O., Pearlman A. L., Two modes of radial migration in early development of the cerebral cortex. Nat. Neurosci. 4, 143–150 (2001). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

