STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation
- PMID: 34215690
- PMCID: PMC8256839
- DOI: 10.1136/jitc-2021-002852
STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation
Abstract
Background: Resistance to an immune checkpoint inhibitor (ICI) is a major obstacle in cancer immunotherapy. The causes of ICI resistance include major histocompatibility complex (MHC)/histocompatibility locus antigen (HLA) class I loss, neoantigen loss, and incomplete antigen presentation. Elimination by natural killer (NK) cells would be expected to be an effective strategy for the treatment of these ICI-resistant tumors. We previously demonstrated that a lipid nanoparticle containing a stimulator of an interferon gene (STING) agonist (STING-LNP) efficiently induced antitumor activity via the activation of NK cells. Thus, we evaluated the potential of reducing ICI resistance by STING-LNPs.
Methods: Lung metastasis of a B16-F10 mouse melanoma was used as an anti-programmed cell death 1 (anti-PD-1)-resistant mouse model. The mice were intravenously injected with the STING-LNP and the mechanism responsible for the improvement of anti-PD-1 resistance by the STING-LNPs was analyzed by RT-qPCR and flow cytometry. The dynamics of STING-LNP were also investigated.
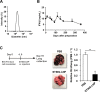
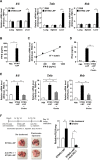
Results: Although anti-PD-1 monotherapy failed to induce an antitumor effect, the combination of the STING-LNP and anti-PD-1 exerted a synergistic antitumor effect. Our results indicate that the STING-LNP treatment significantly increased the expression of CD3, CD4, NK1.1, PD-1 and interferon (IFN)-γ in lung metastases. This change appears to be initiated by the type I IFN produced by liver macrophages that contain the internalized STING-LNPs, leading to the systemic activation of NK cells that express PD-1. The activated NK cells appeared to produce IFN-γ, resulting in an increase in the expression of the PD ligand 1 (PD-L1) in cancer cells, thus leading to a synergistic antitumor effect when anti-PD-1 is administered.
Conclusions: We provide a demonstration to show that a STING-LNP treatment can overcome PD-1 resistance in a B16-F10 lung metastasis model. The mechanism responsible for this indicates that NK cells are activated by stimulating the STING pathway which, in turn, induced the expression of PD-L1 on cancer cells. Based on the findings reported herein, the STING-LNP represents a promising candidate for use in combination therapy with anti-PD-1-resistant tumors.
Keywords: adjuvants; combination; drug therapy; immunotherapy; killer cells; melanoma; natural; pharmaceutic.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: TN and HH received research funding from Ono Pharmaceutical.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
