Long-term open-label continuation study of the safety and efficacy of belimumab for up to 7 years in patients with systemic lupus erythematosus from Japan and South Korea
- PMID: 34215703
- PMCID: PMC8256836
- DOI: 10.1136/rmdopen-2021-001629
Long-term open-label continuation study of the safety and efficacy of belimumab for up to 7 years in patients with systemic lupus erythematosus from Japan and South Korea
Erratum in
-
Correction: Long-term open-label continuation study of the safety and efficacy of belimumab for up to 7 years in patients with systemic lupus erythematosus from japan and south korea.RMD Open. 2024 Jan 12;10(1):e001629corr1. doi: 10.1136/rmdopen-2021-001629corr1. RMD Open. 2024. PMID: 38216292 Free PMC article. No abstract available.
Abstract
Objectives: To evaluate the long-term safety and efficacy of belimumab in patients with systemic lupus erythematosus (SLE) from Japan and South Korea.
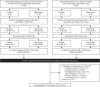
Methods: In this phase III, open-label continuation study (BEL114333; NCT01597622), eligible completers of BEL113750 (NCT01345253) or BEL112341 (NCT01484496) received intravenous belimumab 10 mg/kg every 28 days for ≤7 years. Primary endpoint was safety. Secondary endpoints: SLE Responder Index (SRI)4 response rate, proportion of patients meeting individual SRI4 criteria, SLE flares and prednisone use. Analyses were based on observed data from the first belimumab exposure (either in parent or current study) through to study end.
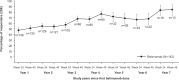
Results: Of 142 enrolled patients who received belimumab, 73.2% completed the study. The study population comprised patients with moderate SLE, mean (SD) Safety of Estrogens in Lupus Erythematosus National Assessment-SLE Disease Activity Index (SELENA-SLEDAI) baseline score of 9.3 (3.9) and 98.6% receiving corticosteroids. Most patients (97.9%) experienced adverse events (AEs); 33.8% experienced serious AEs. Increase in SRI4 (Year 1, Week 24: 47.8%; Year 6, Week 48: 68.2%) and SELENA-SLEDAI responders suggested reductions in disease activity. Proportions of patients with no worsening in Physician Global Assessment/no new organ damage remained stable throughout. Severe SLE flares occurred in 14.8% of patients. Among patients with baseline prednisone-equivalent dose >7.5 mg/day (n=81), the median (min, max) number of days anytime post-baseline that the daily dose was ≤7.5 mg/day or had been reduced by 50% from baseline was 584 (0, 2267).
Conclusions: Favourable safety profile and treatment responses were maintained for ≤7 years in patients with SLE from Japan and South Korea.
Keywords: B-lymphocytes; biological therapy; cytokines; lupus erythematosus; systemic.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: YT received research grants from Mitsubishi-Tanabe, Chugai, AbbVie, Takeda, UCB, Daiichi-Sankyo and Eisai and speaking fees and/or honoraria from Daiichi-Sankyo, Eli Lilly, Novartis, YL Biologics, Bristol-Myers, Eisai, Chugai, AbbVie, Astellas, Pfizer, Sanofi, Asahi-Kasei, GlaxoSmithKline, Mitsubishi-Tanabe, Gilead and Janssen. S-CB has no conflicts of interest to declare. DB, PC, MC, KD, BJ, RK, JL, PM and DAR are employees of GlaxoSmithKline and hold stocks and shares in the company.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
