Rewiring the microbial metabolic network for efficient utilization of mixed carbon sources
- PMID: 34215883
- PMCID: PMC8788776
- DOI: 10.1093/jimb/kuab040
Rewiring the microbial metabolic network for efficient utilization of mixed carbon sources
Abstract
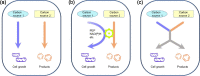
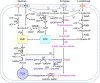
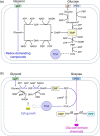
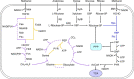
Carbon sources represent the most dominant cost factor in the industrial biomanufacturing of products. Thus, it has attracted much attention to seek cheap and renewable feedstocks, such as lignocellulose, crude glycerol, methanol, and carbon dioxide, for biosynthesis of value-added compounds. Co-utilization of these carbon sources by microorganisms not only can reduce the production cost but also serves as a promising approach to improve the carbon yield. However, co-utilization of mixed carbon sources usually suffers from a low utilization rate. In the past few years, the development of metabolic engineering strategies to enhance carbon source co-utilization efficiency by inactivation of carbon catabolite repression has made significant progress. In this article, we provide informative and comprehensive insights into the co-utilization of two or more carbon sources including glucose, xylose, arabinose, glycerol, and C1 compounds, and we put our focus on parallel utilization, synergetic utilization, and complementary utilization of different carbon sources. Our goal is not only to summarize strategies of co-utilization of carbon sources, but also to discuss how to improve the carbon yield and the titer of target products.
Keywords: Carbon source; Co-utilization; Crude glycerol; Lignocellulosic hydrolysates; Methanol.
© The Author(s) 2021. Published by Oxford University Press on behalf of Society of Industrial Microbiology and Biotechnology.
Figures
References
-
- Bennett R. K., Gonzalez J. E., Whitaker W. B., Antoniewicz M. R., Papoutsakis E. T. (2018). Expression of heterologous non-oxidative pentose phosphate pathway from Bacillus methanolicus and phosphoglucose isomerase deletion improves methanol assimilation and metabolite production by a synthetic Escherichia coli methylotroph. Metabolic Engineering, 45, 75–85. 10.1016/j.ymben.2017.11.016 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

