An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine
- PMID: 34216021
- PMCID: PMC8457105
- DOI: 10.1002/cpt.2350
An Evidence-Based Framework for Evaluating Pharmacogenomics Knowledge for Personalized Medicine
Abstract
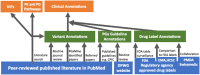
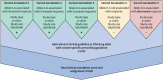
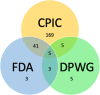
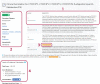
Clinical annotations are one of the most popular resources available on the Pharmacogenomics Knowledgebase (PharmGKB). Each clinical annotation summarizes the association between variant-drug pairs, shows relevant findings from the curated literature, and is assigned a level of evidence (LOE) to indicate the strength of support for that association. Evidence from the pharmacogenomic literature is curated into PharmGKB as variant annotations, which can be used to create new clinical annotations or added to existing clinical annotations. This means that the same clinical annotation can be worked on by multiple curators over time. As more evidence is curated into PharmGKB, the task of maintaining consistency when assessing all the available evidence and assigning an LOE becomes increasingly difficult. To remedy this, a scoring system has been developed to automate LOE assignment to clinical annotations. Variant annotations are scored according to certain attributes, including study size, reported P value, and whether the variant annotation supports or fails to find an association. Clinical guidelines or US Food and Drug Administration (FDA)-approved drug labels which give variant-specific prescribing guidance are also scored. The scores of all annotations attached to a clinical annotation are summed together to give a total score for the clinical annotation, which is used to calculate an LOE. Overall, the system increases transparency, consistency, and reproducibility in LOE assignment to clinical annotations. In combination with increased standardization of how clinical annotations are written, use of this scoring system helps to ensure that PharmGKB clinical annotations continue to be a robust source of pharmacogenomic information.
© 2021 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors report no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

