Pharmacometabolomics identifies candidate predictor metabolites of an L-carnitine treatment mortality benefit in septic shock
- PMID: 34216108
- PMCID: PMC8604225
- DOI: 10.1111/cts.13088
Pharmacometabolomics identifies candidate predictor metabolites of an L-carnitine treatment mortality benefit in septic shock
Abstract
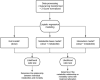
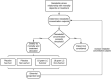
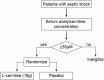
Sepsis-induced metabolic dysfunction contributes to organ failure and death. L-carnitine has shown promise for septic shock, but a recent phase II study of patients with vasopressor-dependent septic shock demonstrated a non-significant reduction in mortality. We undertook a pharmacometabolomics study of these patients (n = 250) to identify metabolic profiles predictive of a 90-day mortality benefit from L-carnitine. The independent predictive value of each pretreatment metabolite concentration, adjusted for L-carnitine dose, on 90-day mortality was determined by logistic regression. A grid-search analysis maximizing the Z-statistic from a binomial proportion test identified specific metabolite threshold levels that discriminated L-carnitine responsive patients. Threshold concentrations were further assessed by hazard ratio and Kaplan-Meier estimate. Accounting for L-carnitine treatment and dose, 11 1 H-NMR metabolites and 12 acylcarnitines were independent predictors of 90-day mortality. Based on the grid-search analysis numerous acylcarnitines and valine were identified as candidate metabolites of drug response. Acetylcarnitine emerged as highly viable for the prediction of an L-carnitine mortality benefit due to its abundance and biological relevance. Using its most statistically significant threshold concentration, patients with pretreatment acetylcarnitine greater than or equal to 35 µM were less likely to die at 90 days if treated with L-carnitine (18 g) versus placebo (p = 0.01 by log rank test). Metabolomics also identified independent predictors of 90-day sepsis mortality. Our proof-of-concept approach shows how pharmacometabolomics could be useful for tackling the heterogeneity of sepsis and informing clinical trial design. In addition, metabolomics can help understand mechanisms of sepsis heterogeneity and variable drug response, because sepsis induces alterations in numerous metabolite concentrations.
© 2021 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures

References
-
- Fleischmann C, Scherag A, Adhikari NKJ, et al. Assessment of global incidence and mortality of hospital‐treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259‐272. - PubMed
-
- Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167‐1174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

