An Observational Cohort Study on the Incidence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection and B.1.1.7 Variant Infection in Healthcare Workers by Antibody and Vaccination Status
- PMID: 34216472
- PMCID: PMC8994591
- DOI: 10.1093/cid/ciab608
An Observational Cohort Study on the Incidence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection and B.1.1.7 Variant Infection in Healthcare Workers by Antibody and Vaccination Status
Abstract
Background: Natural and vaccine-induced immunity will play a key role in controlling the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. SARS-CoV-2 variants have the potential to evade natural and vaccine-induced immunity.
Methods: In a longitudinal cohort study of healthcare workers (HCWs) in Oxfordshire, United Kingdom, we investigated the protection from symptomatic and asymptomatic polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection conferred by vaccination (Pfizer-BioNTech BNT162b2, Oxford-AstraZeneca ChAdOx1 nCOV-19) and prior infection (determined using anti-spike antibody status), using Poisson regression adjusted for age, sex, temporal changes in incidence and role. We estimated protection conferred after 1 versus 2 vaccinations and from infections with the B.1.1.7 variant identified using whole genome sequencing.
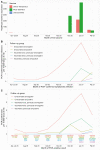
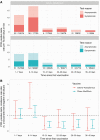
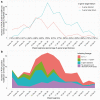
Results: In total, 13 109 HCWs participated; 8285 received the Pfizer-BioNTech vaccine (1407 two doses), and 2738 the Oxford-AstraZeneca vaccine (49 two doses). Compared to unvaccinated seronegative HCWs, natural immunity and 2 vaccination doses provided similar protection against symptomatic infection: no HCW vaccinated twice had symptomatic infection, and incidence was 98% lower in seropositive HCWs (adjusted incidence rate ratio 0.02 [95% confidence interval {CI} < .01-.18]). Two vaccine doses or seropositivity reduced the incidence of any PCR-positive result with or without symptoms by 90% (0.10 [95% CI .02-.38]) and 85% (0.15 [95% CI .08-.26]), respectively. Single-dose vaccination reduced the incidence of symptomatic infection by 67% (0.33 [95% CI .21-.52]) and any PCR-positive result by 64% (0.36 [95% CI .26-.50]). There was no evidence of differences in immunity induced by natural infection and vaccination for infections with S-gene target failure and B.1.1.7.
Conclusions: Natural infection resulting in detectable anti-spike antibodies and 2 vaccine doses both provide robust protection against SARS-CoV-2 infection, including against the B.1.1.7 variant.
Keywords: SARS-CoV-2; antibody; healthcare worker; immunity; vaccine.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
-
- WHO. WHO Coronavirus disease (COVID-19) dashboard. COVID 19 Special Issue 2020; 10. Available at: https://covid19.who.int/. Accessed 1 March 2021.
-
- Hall V, Foulkes S, Charlett A, et al. . Do antibody positive healthcare workers have lower SARS-CoV-2 infection rates than antibody negative healthcare workers? Large multi-centre prospective cohort study (the SIREN study), England: June to November 2020. bioRxiv 2021. doi: 10.1101/2021.01.13.21249642. - DOI
-
- Medicines and Healthcare products Regulatory Agency. MHRA guidance on coronavirus (COVID-19). 2020. Available at: https://www.gov.uk/government/collections/mhra-guidance-on-coronavirus-c.... Accessed 1 March 2021.
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

