Y-27632, a ROCK inhibitor, improved laser-induced shock wave (LISW)-induced cochlear synaptopathy in mice
- PMID: 34217338
- PMCID: PMC8254252
- DOI: 10.1186/s13041-021-00819-1
Y-27632, a ROCK inhibitor, improved laser-induced shock wave (LISW)-induced cochlear synaptopathy in mice
Abstract
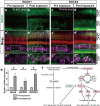
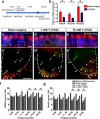
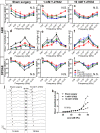
Recently, a pathological condition called cochlear synaptopathy has been clarified, and as a disorder of the auditory nerve synapses that occurs prior to failure of hair cells, it has been recognized as a major cause of sensorineural hearing loss. However, cochlear synaptopathy is untreatable. Inhibition of rho-associated coiled-coil containing protein kinase (ROCK), a serine-threonine protein kinase, has been reported to have neuroprotective and regenerative effects on synaptic pathways in the nervous system, including those in the inner ear. We previously demonstrated the regenerative effect of the ROCK inhibitor, Y-27632, on an excitotoxic cochlear nerve damage model in vitro. In this study, we aimed to validate the effect of ROCK inhibition on mice with cochlear synaptopathy induced by laser-induced shock wave (LISW) in vivo. After the elevation of ROCK1/2 expression in the damaged cochlea was confirmed, we administered Y-27632 locally via the middle ear. The amplitude of wave I in the auditory brainstem response and the number of synapses in the Y-27632-treated cochlea increased significantly. These results clearly demonstrate that ROCK inhibition has a promising clinical application in the treatment of cochlear synaptopathy, which is the major pathology of sensorineural hearing loss.
Keywords: Cochlea; Hearing loss; Inner ear; Regeneration; Rho-associated coiled-coil containing protein kinase (ROCK); Synapse; Y-27632.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

