Evaluation of PCR cycle threshold values by patient population with the quidel lyra SARS-CoV-2 assay
- PMID: 34218165
- PMCID: PMC9761777
- DOI: 10.1016/j.diagmicrobio.2021.115387
Evaluation of PCR cycle threshold values by patient population with the quidel lyra SARS-CoV-2 assay
Abstract
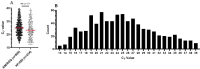
The Lyra SARS-CoV-2 assay was the primary method for molecular testing performed at Barnes-Jewish Healthcare System in St. Louis, Missouri during the initial COVID-19 surge from mid-March to late-April 2020. We performed a retrospective analysis of 1,043 positive Lyra SARS-CoV-2 results during these 36 days to investigate associations between cycle threshold (CT) value and patient characteristics. Total RNA were extracted from NP or OP swabs using either the EasyMag or KingFisher automated extraction systems and quantified with RotorGene Q (Qiagen) or Applied Biosystems 7500 Fast Dx thermocyclers respectively. Notably, we found lower a significant median lower CT for samples tested on the KingFisher-ABI 7500 fastDX (KF/ABI) system compared to the EasyMag/RotorGene (EM/RGQ) platform. Since 77.5% of our tests were ran on the EM/RGQ pipeline we then perform additional analysis on these values and found that C T values in outpatient care settings compared to samples obtained in the emergency department or inpatient had significantly lower C T values. These collective findings suggests a difference in viral load amongst various patient populations.
Keywords: Clinical virology; Molecular assays; SARS-CoV-2.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest There are no conflicts of interest.
Figures


Similar articles
-
Lack of correlation between the SARS-CoV-2 cycle threshold (Ct ) value and clinical outcomes in patients with COVID-19.J Med Virol. 2021 Oct;93(10):6059-6062. doi: 10.1002/jmv.27171. Epub 2021 Jul 6. J Med Virol. 2021. PMID: 34196409 Free PMC article.
-
A Multiplex One-Step RT-qPCR Protocol to Detect SARS-CoV-2 in NP/OP Swabs and Saliva.Curr Protoc. 2021 May;1(5):e145. doi: 10.1002/cpz1.145. Curr Protoc. 2021. PMID: 34004070 Free PMC article.
-
Combined throat/nasal swab sampling for SARS-CoV-2 is equivalent to nasopharyngeal sampling.Eur J Clin Microbiol Infect Dis. 2021 Jan;40(1):193-195. doi: 10.1007/s10096-020-03972-y. Epub 2020 Jul 14. Eur J Clin Microbiol Infect Dis. 2021. PMID: 32666481 Free PMC article.
-
Caution in interpretation of SARS-CoV-2 quantification based on RT-PCR cycle threshold value.Diagn Microbiol Infect Dis. 2021 Jul;100(3):115366. doi: 10.1016/j.diagmicrobio.2021.115366. Epub 2021 Mar 4. Diagn Microbiol Infect Dis. 2021. PMID: 33756311 Free PMC article.
-
Efficacy comparison of three rapid antigen tests for SARS-CoV-2 and how viral load impact their performance.J Med Virol. 2021 Oct;93(10):5783-5788. doi: 10.1002/jmv.27108. Epub 2021 Jun 4. J Med Virol. 2021. PMID: 34050945 Free PMC article.
Cited by
-
Generation of quality-controlled SARS-CoV-2 variant stocks.Nat Protoc. 2023 Dec;18(12):3821-3855. doi: 10.1038/s41596-023-00897-6. Epub 2023 Oct 13. Nat Protoc. 2023. PMID: 37833423 Review.
-
Isolation of SARS-CoV-2 in Viral Cell Culture in Immunocompromised Patients With Persistently Positive RT-PCR Results.Front Cell Infect Microbiol. 2022 Feb 2;12:804175. doi: 10.3389/fcimb.2022.804175. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35186791 Free PMC article.
References
-
- . Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med. 2020;26:861–868. - PubMed
-
- Fuller JA, Njenga MK, Bigogo G, Aura B, Ope MO, Nderitu L, et al. Association of the CT values of real-time PCR of viral upper respiratory tract infection with clinical severity, Kenya. J Med Virol. 2013;85:924–932. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

