Intra-therapeutic dosimetry of [177Lu]Lu-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer patients and correlation with treatment outcome
- PMID: 34218300
- PMCID: PMC8803803
- DOI: 10.1007/s00259-021-05471-4
Intra-therapeutic dosimetry of [177Lu]Lu-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer patients and correlation with treatment outcome
Abstract
Introduction: While [177Lu]Lu-PSMA radioligand therapy is currently only applied in end-stage metastatic castrate-resistant prostate cancer (mCRPC) patients, also low-volume hormone-sensitive metastatic prostate cancer (mHSPC) patients can benefit from it. However, there are toxicity concerns related to the sink effect in low-volume disease. This prospective study aims to determine the kinetics of [177Lu]Lu-PSMA in mHSPC patients, analyzing the doses to organs at risk (salivary glands, kidneys, liver, and bone marrow) and tumor lesions < 1 cm diameter.
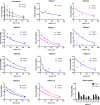
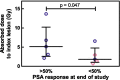
Methods: Ten mHSPC patients underwent two cycles of [177Lu]Lu-PSMA therapy. Three-bed position SPECT/CT was performed at 5 time points after each therapy. Organ dosimetry and lesion dosimetry were performed using commercial software and a manual approach, respectively. Correlation between absorbed index lesion dose and treatment response (PSA drop of > 50% at the end of the study) was calculated and given as Spearman's r and p-values.
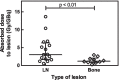
Results: Kinetics of [177Lu]Lu-PSMA in mHSPC patients are comparable to those in mCRPC patients. Lesion absorbed dose was high (3.25 ± 3.19 Gy/GBq) compared to organ absorbed dose (salivary glands: 0.39 ± 0.17 Gy/GBq, kidneys: 0.49 ± 0.11 Gy/GBq, liver: 0.09 ± 0.01 Gy/GBq, bone marrow: 0.017 ± 0.008 Gy/GBq). A statistically significant correlation was found between treatment response and absorbed index lesion dose (p = 0.047).
Conclusions: We successfully performed small lesion dosimetry and showed that the tumor sink effect in mHSPC patients is of less concern than was expected. Tumor-to-organ ratio of absorbed dose was high and tumor uptake correlates with PSA response. Additional treatment cycles are legitimate in terms of organ toxicity and could lead to better tumor response.
Keywords: Dosimetry; Prostate cancer; Radionuclide therapy; [177Lu]Lu-PSMA; mHSPC.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. - PubMed
-
- James ND, Spears MR, Clarke NW, Dearnaley DP, De Bono JS, Gale J, et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019) Eur Urol. 2015;67:1028–1038. - PubMed
-
- Wright GL, Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

