Perspectives on the development and future of oocyte IVM in clinical practice
- PMID: 34218388
- PMCID: PMC8266966
- DOI: 10.1007/s10815-021-02263-5
Perspectives on the development and future of oocyte IVM in clinical practice
Abstract
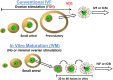
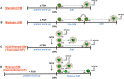
Oocyte in vitro maturation (IVM) is an assisted reproductive technology designed to obtain mature oocytes following culture of immature cumulus-oocyte complexes collected from antral follicles. Although IVM has been practiced for decades and is no longer considered experimental, the uptake of IVM in clinical practice is currently limited. The purpose of this review is to ensure reproductive medicine professionals understand the appropriate use of IVM drawn from the best available evidence supporting its clinical potential and safety in selected patient groups. This group of scientists and fertility specialists, with expertise in IVM in the ART laboratory and/or clinic, explore here the development of IVM towards acquisition of a non-experimental status and, in addition, critically appraise the current and future role of IVM in human ART.
Keywords: Fertility preservation; In vitro maturation (IVM); Onco-fertility; Oocyte maturation; Polycystic ovary syndrome (PCOS).
Figures


References
-
- Pincus G, Saunders B. The comparative behavior of mammalian eggs in vivo and in vitro. VI. The maturation of human ovarian ova. Anat Rec. 1939;75:537–545.
-
- Rock J, Menkin MF. In vitro fertilization and cleavage of human ovarian eggs. Science. 1944;100:105–107. - PubMed
-
- Menkin MF, Rock J. In vitro fertilization and cleavage of human ovarian eggs. Am J Obstet Gynecol. 1948;55:440–452. - PubMed
-
- Thompson JG, Gilchrist RB. Pioneering contributions by Robert Edwards to oocyte in vitro maturation (IVM) Mol Hum Reprod. 2013;19:794–798. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

