Model-based meta-analysis of changes in circulatory system physiology in patients with chronic heart failure
- PMID: 34218511
- PMCID: PMC8452295
- DOI: 10.1002/psp4.12676
Model-based meta-analysis of changes in circulatory system physiology in patients with chronic heart failure
Abstract
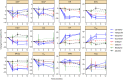
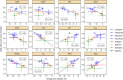
To characterize and compare various medicines for chronic heart failure (CHF), changes in circulatory physiological parameter during pharmacotherapy were investigated by a model-based meta-analysis (MBMA) of circulatory physiology. The clinical data from 61 studies mostly in patients with heart failure with reduced ejection fraction (HFrEF), reporting changes in heart rate, blood pressure, or ventricular volumes after treatment with carvedilol, metoprolol, bisoprolol, bucindolol, enalapril, aliskiren, or felodipine, were analyzed. Seven cardiac and vasculature function indices were estimated without invasive measurements using models based on appropriate assumptions, and their correlations with the mortality were assessed. Estimated myocardial oxygen consumption, a cardiac load index, correlated excellently with the mortality at 3, 6, and 12 months after treatment initiation, and it explained differences in mortality across the different medications. The analysis based on the present models were reasonably consistent with the hypothesis that the treatment of HFrEF with various medications is due to effectively reducing the cardiac load. Assessment of circulatory physiological parameters by using MBMA would be insightful for quantitative understanding of CHF treatment.
© 2021 The Authors. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Y.S. is an employee of Sanofi K.K. However, Sanofi K.K. is not involved in this analysis. S.G. was involved in this study before she joined Astellas Pharma Inc. All other authors declared no competing interests for this work.
Figures


Similar articles
-
Efficacy and safety of bisoprolol fumarate compared with carvedilol in Japanese patients with chronic heart failure: results of the randomized, controlled, double-blind, Multistep Administration of bisoprolol IN Chronic Heart Failure II (MAIN-CHF II) study.Heart Vessels. 2014 Mar;29(2):238-47. doi: 10.1007/s00380-013-0340-3. Epub 2013 Apr 5. Heart Vessels. 2014. PMID: 23559359 Clinical Trial.
-
The mortality benefit of carvedilol versus bisoprolol in patients with heart failure with reduced ejection fraction.Korean J Intern Med. 2019 Sep;34(5):1030-1039. doi: 10.3904/kjim.2018.009. Epub 2018 Oct 16. Korean J Intern Med. 2019. PMID: 30317846 Free PMC article.
-
Differences between beta-blockers in patients with chronic heart failure and chronic obstructive pulmonary disease: a randomized crossover trial.J Am Coll Cardiol. 2010 Apr 27;55(17):1780-7. doi: 10.1016/j.jacc.2010.01.024. J Am Coll Cardiol. 2010. PMID: 20413026 Clinical Trial.
-
Beta-blocker benefit according to severity of heart failure.Eur J Heart Fail. 2003 Jun;5(3):281-9. doi: 10.1016/s1388-9842(03)00042-4. Eur J Heart Fail. 2003. PMID: 12798825 Review.
-
Ivabradine Improves Cardiac Function and Increases Exercise Capacity in Patients with Chronic Heart Failure.Int Heart J. 2019 Jul 27;60(4):899-909. doi: 10.1536/ihj.18-559. Epub 2019 Jul 12. Int Heart J. 2019. PMID: 31308326
Cited by
-
Exercise training outcomes in patients with chronic heart failure with reduced ejection fraction depend on patient background.Front Cardiovasc Med. 2024 Jan 31;11:1330235. doi: 10.3389/fcvm.2024.1330235. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38361589 Free PMC article.
-
Effect of ICD/CRT-D Implantation on Adverse Events and Readmission Rate in Patients with Chronic Heart Failure (CHF).Comput Math Methods Med. 2022 May 16;2022:8695291. doi: 10.1155/2022/8695291. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Jun 28;2023:9759310. doi: 10.1155/2023/9759310. PMID: 35615439 Free PMC article. Retracted. Clinical Trial.
References
-
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137‐e161. - PubMed
-
- Williams B, Mancia G, Spiering W, et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021‐3104. - PubMed
-
- Poole‐Wilson PA, Swedberg K, Cleland JGF, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7‐13. - PubMed
-
- MERIT‐HF Study Group . Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT‐HF). Lancet. 1999;353:2001‐2007. - PubMed
-
- CIBIS‐II Investigators and Committees . The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet. 1999;353:9‐13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

