Influenza immune escape under heterogeneous host immune histories
- PMID: 34218981
- PMCID: PMC8578193
- DOI: 10.1016/j.tim.2021.05.009
Influenza immune escape under heterogeneous host immune histories
Abstract
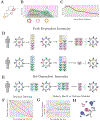
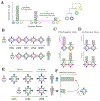
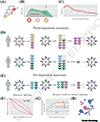
In a pattern called immune imprinting, individuals gain the strongest immune protection against the influenza strains encountered earliest in life. In many recent examples, differences in early infection history can explain birth year-associated differences in susceptibility (cohort effects). Susceptibility shapes strain fitness, but without a clear conceptual model linking host susceptibility to the identity and order of past infections general conclusions on the evolutionary and epidemic implications of cohort effects are not possible. Failure to differentiate between cohort effects caused by differences in the set, rather than the order (path), of past infections is a current source of confusion. We review and refine hypotheses for path-dependent cohort effects, which include imprinting. We highlight strategies to measure their underlying causes and emergent consequences.
Keywords: antigenic evolution; cohort effects; immune escape; immune imprinting; influenza; original antigenic sin.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests There are no interests to declare.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

