Role of osteoclasts in oral homeostasis and jawbone diseases
- PMID: 34220275
- PMCID: PMC8248583
- DOI: 10.1002/osi2.1078
Role of osteoclasts in oral homeostasis and jawbone diseases
Abstract
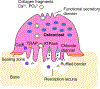
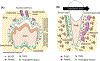
The jawbone is a unique structure as it serves multiple functions in mastication. Given the fact that the jawbone is remodeled faster than other skeletal bones, bone cells in the jawbone may respond differently to local and systemic cues to regulate bone remodeling and adaptation. Osteoclasts are bone cells responsible for removing old bone, playing an essential role in bone remodeling. Although bone resorption by osteoclasts is required for dental tissue development, homeostasis and repair, excessive osteoclast activity is associated with oral skeletal diseases such as periodontitis. In addition, antiresorptive medications used to prevent bone homeostasis of tumors can cause osteonecrosis of the jaws that is a major concern to the dentist. Therefore, understanding of the role of osteoclasts in oral homeostasis under physiological and pathological conditions leads to better targeted therapeutic options for skeletal diseases to maintain patients' oral health. Here, we highlight the unique features of the jawbone compared to the long bone and the involvement of osteoclasts in the jawbone-specific diseases.
Keywords: bone remodeling; jawbone; mechanical stress; osteoclast; osteonecrosis.
Conflict of interest statement
DISCLOSURES All authors declare no conflicts of interest.
Figures




References
-
- Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, Oda R, et al. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005;20(3):399–409. - PubMed
-
- Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38(6):758–68. - PubMed
-
- Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11(2):76–81. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
