A Retrospective Case Series Analysis of the Relationship Between Phenylalanine: Tyrosine Ratio and Cerebral Glucose Metabolism in Classical Phenylketonuria and Hyperphenylalaninemia
- PMID: 34220424
- PMCID: PMC8248344
- DOI: 10.3389/fnins.2021.664525
A Retrospective Case Series Analysis of the Relationship Between Phenylalanine: Tyrosine Ratio and Cerebral Glucose Metabolism in Classical Phenylketonuria and Hyperphenylalaninemia
Abstract
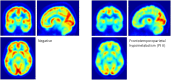
We retrospectively examined the relationship between blood biomarkers, in particular the historical mean phenylalanine to tyrosine (Phe:Tyr) ratio, and cerebral glucose metabolism. We hypothesized that the historical mean Phe:Tyr ratio would be more predictive of cerebral glucose metabolism than the phenylalanine (Phe) level alone. We performed a retrospective case series analysis involving 11 adult classical phenylketonuria/hyperphenylalaninemia patients under the care of an Inherited Metabolic & Neuropsychiatry Clinic who had complained of memory problems, collating casenote data from blood biochemistry, and clinical [18F]fluorodeoxyglucose positron emission tomography ([18F]FDG PET). The Phe:Tyr ratio was calculated for individual blood samples and summarized as historical mean Phe:Tyr ratio (Phe:Tyr) and historical standard deviation in Phe:Tyr ratio (SD-Phe:Tyr), for each patient. Visual analyses of [18F]FDG PET revealed heterogeneous patterns of glucose hypometabolism for eight patients. [18F]FDG PET standardized uptake was negatively correlated with Phe in a large cluster with peak localized to right superior parietal gyrus. Even larger clusters of negative correlation that encompassed most of the brain, with frontal peaks, were observed with Phe:Tyr, and SD-Phe:Tyr. Our case series analysis provides further evidence for the association between blood biomarkers, and cerebral glucose hypometabolism. Mean historical blood Phe:Tyr ratio, and its standard deviation over time, appear to be more indicative of global cerebral glucose metabolism in patients with memory problems than Phe.
Keywords: [18F]fluorodeoxyglucose PET; hyperphenylalaninemia; intellectual function; phenylalanine:tyrosine ratio; phenylketonuria.
Copyright © 2021 McGinnity, Riaño Barros, Guedj, Girard, Symeon, Walker, Barrington, Summers, Pitkanen and Rahman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Data on phenylalanine-to-tyrosine ratios in assessment of tetrahydrobiopterin (BH4)-responsiveness in patients with hyperphenylalaninemia.Data Brief. 2022 Feb 4;41:107926. doi: 10.1016/j.dib.2022.107926. eCollection 2022 Apr. Data Brief. 2022. PMID: 35198697 Free PMC article.
-
Use of phenylalanine-to-tyrosine ratio determined by tandem mass spectrometry to improve newborn screening for phenylketonuria of early discharge specimens collected in the first 24 hours.Clin Chem. 1998 Dec;44(12):2405-9. Clin Chem. 1998. PMID: 9836704
-
Comparison of tandem mass spectrometry and amino acid analyzer for phenylalanine and tyrosine monitoring--implications for clinical management of patients with hyperphenylalaninemia.Clin Biochem. 2015 Jan;48(1-2):14-8. doi: 10.1016/j.clinbiochem.2014.09.014. Epub 2014 Sep 28. Clin Biochem. 2015. PMID: 25261586
-
Phenylketonuria oxidative stress and energy dysregulation: Emerging pathophysiological elements provide interventional opportunity.Mol Genet Metab. 2022 Jun;136(2):111-117. doi: 10.1016/j.ymgme.2022.03.012. Epub 2022 Mar 29. Mol Genet Metab. 2022. PMID: 35379539 Free PMC article. Review.
-
Clinical, genetic, and experimental research of hyperphenylalaninemia.Front Genet. 2023 Jan 4;13:1051153. doi: 10.3389/fgene.2022.1051153. eCollection 2022. Front Genet. 2023. PMID: 36685931 Free PMC article. Review.
Cited by
-
Metabolomic changes in children with autism.World J Clin Pediatr. 2024 Jun 9;13(2):92737. doi: 10.5409/wjcp.v13.i2.92737. eCollection 2024 Jun 9. World J Clin Pediatr. 2024. PMID: 38947988 Free PMC article.
-
Engineering Organoids for in vitro Modeling of Phenylketonuria.Front Mol Neurosci. 2022 Jan 10;14:787242. doi: 10.3389/fnmol.2021.787242. eCollection 2021. Front Mol Neurosci. 2022. PMID: 35082602 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

