The Potential Role of Inflammation in Modulating Endogenous Hippocampal Neurogenesis After Spinal Cord Injury
- PMID: 34220440
- PMCID: PMC8249862
- DOI: 10.3389/fnins.2021.682259
The Potential Role of Inflammation in Modulating Endogenous Hippocampal Neurogenesis After Spinal Cord Injury
Abstract
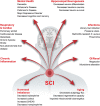
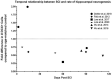
Currently there are approximately 291,000 people suffering from a spinal cord injury (SCI) in the United States. SCI is associated with traumatic changes in mobility and neuralgia, as well as many other long-term chronic health complications, including metabolic disorders, diabetes mellitus, non-alcoholic steatohepatitis, osteoporosis, and elevated inflammatory markers. Due to medical advances, patients with SCI survive much longer than previously. This increase in life expectancy exposes them to novel neurological complications such as memory loss, cognitive decline, depression, and Alzheimer's disease. In fact, these usually age-associated disorders are more prevalent in people living with SCI. A common factor of these disorders is the reduction in hippocampal neurogenesis. Inflammation, which is elevated after SCI, plays a major role in modulating hippocampal neurogenesis. While there is no clear consensus on the mechanism of the decline in hippocampal neurogenesis and cognition after SCI, we will examine in this review how SCI-induced inflammation could modulate hippocampal neurogenesis and provoke age-associated neurological disorders. Thereafter, we will discuss possible therapeutic options which may mitigate the influence of SCI associated complications on hippocampal neurogenesis.
Keywords: inflammation; memory and cognitive impairment; neurogenesis; spinal cord injury; therapeutics.
Copyright © 2021 Sefiani and Geoffroy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Depression-like behavior is associated with deficits in cognition and hippocampal neurogenesis in a subset of spinally contused male, but not female, rats.Brain Behav Immun. 2025 Jan;123:270-287. doi: 10.1016/j.bbi.2024.09.015. Epub 2024 Sep 15. Brain Behav Immun. 2025. PMID: 39288895
-
Potential role of hippocampal neurogenesis in spinal cord injury induced post-trauma depression.Neural Regen Res. 2024 Oct 1;19(10):2144-2156. doi: 10.4103/1673-5374.392855. Epub 2024 Jan 8. Neural Regen Res. 2024. PMID: 38488549 Free PMC article.
-
Endoplasmic Reticulum Stress and Disrupted Neurogenesis in the Brain Are Associated with Cognitive Impairment and Depressive-Like Behavior after Spinal Cord Injury.J Neurotrauma. 2016 Nov 1;33(21):1919-1935. doi: 10.1089/neu.2015.4348. Epub 2016 May 16. J Neurotrauma. 2016. PMID: 27050417 Free PMC article.
-
Harnessing neurogenesis in the adult brain-A role in type 2 diabetes mellitus and Alzheimer's disease.Int Rev Neurobiol. 2020;155:235-269. doi: 10.1016/bs.irn.2020.03.020. Int Rev Neurobiol. 2020. PMID: 32854856 Free PMC article. Review.
-
Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: can exercise compensate?Neurosci Biobehav Rev. 2016 Feb;61:121-31. doi: 10.1016/j.neubiorev.2015.12.004. Epub 2015 Dec 13. Neurosci Biobehav Rev. 2016. PMID: 26695382 Review.
Cited by
-
Frontiers in Neurogenesis.Cells. 2022 Nov 11;11(22):3567. doi: 10.3390/cells11223567. Cells. 2022. PMID: 36428996 Free PMC article.
-
Coexistence of chronic hyperalgesia and multilevel neuroinflammatory responses after experimental SCI: a systematic approach to profiling neuropathic pain.J Neuroinflammation. 2022 Oct 29;19(1):264. doi: 10.1186/s12974-022-02628-2. J Neuroinflammation. 2022. PMID: 36309729 Free PMC article.
-
Targeting neuroinflammation: 3-monothiopomalidomide a new drug candidate to mitigate traumatic brain injury and neurodegeneration.J Biomed Sci. 2025 Jun 16;32(1):57. doi: 10.1186/s12929-025-01150-w. J Biomed Sci. 2025. PMID: 40524167 Free PMC article.
-
Spinal cord injury leads to more neurodegeneration in the hippocampus of aged male rats compared to young rats.Exp Brain Res. 2023 Jun;241(6):1569-1583. doi: 10.1007/s00221-023-06577-x. Epub 2023 May 2. Exp Brain Res. 2023. PMID: 37129669
-
Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Accelerate Functional Recovery After Spinal Cord Injury by Promoting the Phagocytosis of Macrophages to Clean Myelin Debris.Front Cell Dev Biol. 2021 Nov 8;9:772205. doi: 10.3389/fcell.2021.772205. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34820385 Free PMC article.
References
-
- Aid S., Silva A. C., Candelario-Jalil E., Choi S. H., Rosenberg G. A., Bosetti F. (2010). Cyclooxygenase-1 and -2 differentially modulate lipopolysaccharide-induced blood-brain barrier disruption through matrix metalloproteinase activity. J. Cereb. Blood Flow Metab. 30 370–380. 10.1038/jcbfm.2009.223 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

