Broad Influence of Mutant Ataxin-3 on the Proteome of the Adult Brain, Young Neurons, and Axons Reveals Central Molecular Processes and Biomarkers in SCA3/MJD Using Knock-In Mouse Model
- PMID: 34220448
- PMCID: PMC8248683
- DOI: 10.3389/fnmol.2021.658339
Broad Influence of Mutant Ataxin-3 on the Proteome of the Adult Brain, Young Neurons, and Axons Reveals Central Molecular Processes and Biomarkers in SCA3/MJD Using Knock-In Mouse Model
Abstract
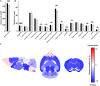
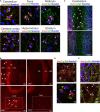
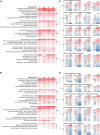
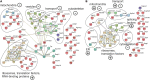
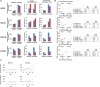
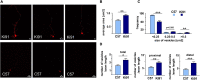
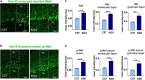
Spinocerebellar ataxia type 3 (SCA3/MJD) is caused by CAG expansion mutation resulting in a long polyQ domain in mutant ataxin-3. The mutant protein is a special type of protease, deubiquitinase, which may indicate its prominent impact on the regulation of cellular proteins levels and activity. Yet, the global model picture of SCA3 disease progression on the protein level, molecular pathways in the brain, and neurons, is largely unknown. Here, we investigated the molecular SCA3 mechanism using an interdisciplinary research paradigm combining behavioral and molecular aspects of SCA3 in the knock-in ki91 model. We used the behavior, brain magnetic resonance imaging (MRI) and brain tissue examination to correlate the disease stages with brain proteomics, precise axonal proteomics, neuronal energy recordings, and labeling of vesicles. We have demonstrated that altered metabolic and mitochondrial proteins in the brain and the lack of weight gain in Ki91 SCA3/MJD mice is reflected by the failure of energy metabolism recorded in neonatal SCA3 cerebellar neurons. We have determined that further, during disease progression, proteins responsible for metabolism, cytoskeletal architecture, vesicular, and axonal transport are disturbed, revealing axons as one of the essential cell compartments in SCA3 pathogenesis. Therefore we focus on SCA3 pathogenesis in axonal and somatodendritic compartments revealing highly increased axonal localization of protein synthesis machinery, including ribosomes, translation factors, and RNA binding proteins, while the level of proteins responsible for cellular transport and mitochondria was decreased. We demonstrate the accumulation of axonal vesicles in neonatal SCA3 cerebellar neurons and increased phosphorylation of SMI-312 positive adult cerebellar axons, which indicate axonal dysfunction in SCA3. In summary, the SCA3 disease mechanism is based on the broad influence of mutant ataxin-3 on the neuronal proteome. Processes central in our SCA3 model include disturbed localization of proteins between axonal and somatodendritic compartment, early neuronal energy deficit, altered neuronal cytoskeletal structure, an overabundance of various components of protein synthesis machinery in axons.
Keywords: Machado-Joseph disease (MJD); ataxin-3; axon; energy metabolism; neurodegenerative; proteome; spinocerebellar ataxia type 3 (SCA3); vesicular transport.
Copyright © 2021 Wiatr, Marczak, Pérot, Brouillet, Flament and Figiel.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Altered Levels of Proteins and Phosphoproteins, in the Absence of Early Causative Transcriptional Changes, Shape the Molecular Pathogenesis in the Brain of Young Presymptomatic Ki91 SCA3/MJD Mouse.Mol Neurobiol. 2019 Dec;56(12):8168-8202. doi: 10.1007/s12035-019-01643-4. Epub 2019 Jun 14. Mol Neurobiol. 2019. PMID: 31201651 Free PMC article.
-
A new humanized ataxin-3 knock-in mouse model combines the genetic features, pathogenesis of neurons and glia and late disease onset of SCA3/MJD.Neurobiol Dis. 2015 Jan;73:174-88. doi: 10.1016/j.nbd.2014.09.020. Epub 2014 Oct 7. Neurobiol Dis. 2015. PMID: 25301414
-
Frequency of SCA1, SCA2, SCA3/MJD, SCA6, SCA7, and DRPLA CAG trinucleotide repeat expansion in patients with hereditary spinocerebellar ataxia from Chinese kindreds.Arch Neurol. 2000 Apr;57(4):540-4. doi: 10.1001/archneur.57.4.540. Arch Neurol. 2000. PMID: 10768629
-
Toward therapeutic targets for SCA3: Insight into the role of Machado-Joseph disease protein ataxin-3 in misfolded proteins clearance.Prog Neurobiol. 2015 Sep;132:34-58. doi: 10.1016/j.pneurobio.2015.06.004. Epub 2015 Jun 27. Prog Neurobiol. 2015. PMID: 26123252 Review.
-
Experimental and Clinical Strategies for Treating Spinocerebellar Ataxia Type 3.Neuroscience. 2018 Feb 10;371:138-154. doi: 10.1016/j.neuroscience.2017.11.051. Epub 2017 Dec 8. Neuroscience. 2018. PMID: 29229556 Review.
Cited by
-
Mitochondrial Dysfunction in Spinocerebellar Ataxia Type 3 Is Linked to VDAC1 Deubiquitination.Int J Mol Sci. 2022 May 25;23(11):5933. doi: 10.3390/ijms23115933. Int J Mol Sci. 2022. PMID: 35682609 Free PMC article.
-
Predicting Which Mitophagy Proteins Are Dysregulated in Spinocerebellar Ataxia Type 3 (SCA3) Using the Auto-p2docking Pipeline.Int J Mol Sci. 2025 Feb 4;26(3):1325. doi: 10.3390/ijms26031325. Int J Mol Sci. 2025. PMID: 39941093 Free PMC article.
-
Selective transduction of cerebellar Purkinje and granule neurons using delivery of AAV-PHP.eB and AAVrh10 vectors at axonal terminal locations.Front Mol Neurosci. 2022 Sep 13;15:947490. doi: 10.3389/fnmol.2022.947490. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36176957 Free PMC article.
-
Longitudinal MRI and 1H-MRS study of SCA7 mouse forebrain reveals progressive multiregional atrophy and early brain metabolite changes indicating early neuronal and glial dysfunction.PLoS One. 2024 Jan 16;19(1):e0296790. doi: 10.1371/journal.pone.0296790. eCollection 2024. PLoS One. 2024. PMID: 38227598 Free PMC article.
-
Glutamatergic Synapse Dysfunction in Drosophila Neuromuscular Junctions Can Be Rescued by Proteostasis Modulation.Front Mol Neurosci. 2022 Jul 15;15:842772. doi: 10.3389/fnmol.2022.842772. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35909443 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

