Myricetin Inhibits SARS-CoV-2 Viral Replication by Targeting Mpro and Ameliorates Pulmonary Inflammation
- PMID: 34220507
- PMCID: PMC8248548
- DOI: 10.3389/fphar.2021.669642
Myricetin Inhibits SARS-CoV-2 Viral Replication by Targeting Mpro and Ameliorates Pulmonary Inflammation
Abstract
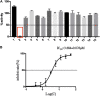
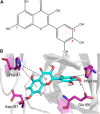
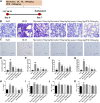
The coronavirus disease 2019 (COVID-19) has spread widely around the world and has seriously affected the human health of tens of millions of people. In view of lacking anti-virus drugs target to SARS-CoV-2, there is an urgent need to develop effective new drugs. In this study, we reported our discovery of SARS-CoV-2 Mpro inhibitors. We selected 15 natural compounds, including 7 flavonoids, 3 coumarins, 2 terpenoids, one henolic, one aldehyde and one steroid compound for molecular docking and enzymatic screening. Myricetin were identified to have potent inhibit activity with IC50 3.684 ± 0.076 μM in the enzyme assay. The binding pose of Myricetin with SARS-CoV-2 Mpro was identified using molecular docking method. In the binding pocket of SARS-CoV-2 Mpro, the chromone ring of Myricetin interacts with His41 through π-π stacking, and the 3'-, 4'- and 7-hydroxyl of Myricetin interact with Phe140, Glu166and Asp187 through hydrogen bonds. Significantly, our results showed that Myricetin has potent effect on bleomycin-induced pulmonary inflammation by inhibiting the infiltration of inflammatory cells and the secretion of inflammatory cytokines IL-6, IL-1α, TNF-α and IFN-γ. Overall, Myricetin may be a potential drug for anti-virus and symptomatic treatment of COVID-19.
Keywords: 3CLpro (Mpro); COVID-19; SARS-CoV-2; myricetin; pulmonary inflammation.
Copyright © 2021 Xiao, Cui, Zheng, Wang, Sun, Gao, Bao, Ren, Yang, Lin, Li, Li, Yang and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Effect of dihydromyricetin on SARS-CoV-2 viral replication and pulmonary inflammation and fibrosis.Phytomedicine. 2021 Oct;91:153704. doi: 10.1016/j.phymed.2021.153704. Epub 2021 Aug 8. Phytomedicine. 2021. PMID: 34419736 Free PMC article.
-
Commercially Available Flavonols Are Better SARS-CoV-2 Inhibitors than Isoflavone and Flavones.Viruses. 2022 Jun 30;14(7):1458. doi: 10.3390/v14071458. Viruses. 2022. PMID: 35891437 Free PMC article.
-
Molecular Docking Studies on the Anti-viral Effects of Compounds From Kabasura Kudineer on SARS-CoV-2 3CLpro.Front Mol Biosci. 2020 Dec 23;7:613401. doi: 10.3389/fmolb.2020.613401. eCollection 2020. Front Mol Biosci. 2020. PMID: 33425994 Free PMC article.
-
Investigating novel thiazolyl-indazole derivatives as scaffolds for SARS-CoV-2 MPro inhibitors.Eur J Med Chem Rep. 2022 Apr;4:100034. doi: 10.1016/j.ejmcr.2022.100034. Epub 2022 Feb 10. Eur J Med Chem Rep. 2022. PMID: 37519829 Free PMC article.
-
2-Pyridone natural products as inhibitors of SARS-CoV-2 main protease.Chem Biol Interact. 2021 Feb 1;335:109348. doi: 10.1016/j.cbi.2020.109348. Epub 2020 Dec 2. Chem Biol Interact. 2021. PMID: 33278462 Free PMC article.
Cited by
-
Methodology-Centered Review of Molecular Modeling, Simulation, and Prediction of SARS-CoV-2.Chem Rev. 2022 Jul 13;122(13):11287-11368. doi: 10.1021/acs.chemrev.1c00965. Epub 2022 May 20. Chem Rev. 2022. PMID: 35594413 Free PMC article. Review.
-
Screening of Mpro Protease (SARS-CoV-2) Covalent Inhibitors from an Anthocyanin-Rich Blueberry Extract Using an HRMS-Based Analytical Platform.Molecules. 2024 Jun 6;29(11):2702. doi: 10.3390/molecules29112702. Molecules. 2024. PMID: 38893578 Free PMC article.
-
Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance.Life (Basel). 2023 Apr 4;13(4):948. doi: 10.3390/life13040948. Life (Basel). 2023. PMID: 37109477 Free PMC article. Review.
-
Promising Antiviral Activities of Natural Flavonoids against SARS-CoV-2 Targets: Systematic Review.Int J Mol Sci. 2021 Oct 14;22(20):11069. doi: 10.3390/ijms222011069. Int J Mol Sci. 2021. PMID: 34681727 Free PMC article.
-
Oxidative stress and COVID-19-associated neuronal dysfunction: mechanisms and therapeutic implications.Acta Biochim Biophys Sin (Shanghai). 2023 Jun 25;55(8):1153-1167. doi: 10.3724/abbs.2023085. Acta Biochim Biophys Sin (Shanghai). 2023. PMID: 37357527 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

