He-Jie-Shen-Shi Decoction as an Adjuvant Therapy on Severe Coronavirus Disease 2019: A Retrospective Cohort and Potential Mechanistic Study
- PMID: 34220524
- PMCID: PMC8250425
- DOI: 10.3389/fphar.2021.700498
He-Jie-Shen-Shi Decoction as an Adjuvant Therapy on Severe Coronavirus Disease 2019: A Retrospective Cohort and Potential Mechanistic Study
Abstract
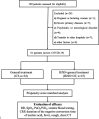
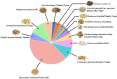
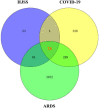
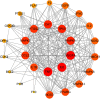
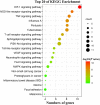
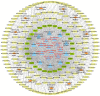
Combination therapy using Western and traditional Chinese medicines has shown notable effects on coronavirus disease 2019 (COVID-19). The He-Jie-Shen-Shi decoction (HJSS), composed of Bupleurum chinense DC., Scutellaria baicalensis Georgi, Pinellia ternata (Thunb.) Makino, Glycyrrhiza uralensis Fisch. ex DC., and nine other herbs, has been used to treat severe COVID-19 in clinical practice. The aim of this study was to compare the clinical efficacies of HJSS combination therapy and Western monotherapy against severe COVID-19 and to study the potential action mechanism of HJSS. From February 2020 to March 2020, 81 patients with severe COVID-19 in Wuhan Tongji Hospital were selected for retrospective cohort study. Network pharmacology was conducted to predict the possible mechanism of HJSS on COVID-19-related acute respiratory distress syndrome (ARDS). Targets of active components in HJSS were screened using the Traditional Chinese Medicine Systems Pharmacology (TCMSP) and PharmMapper databases. The targets of COVID-19 and ARDS were obtained from GeneCards and Online Mendelian Inheritance in Man databases. The key targets of HJSS in COVID-19 and ARDS were obtained based on the protein-protein interaction network (PPI). Kyoto Encyclopedia of Genes and Genomes analysis (KEGG) was conducted to predict the pathways related to the targets of HJSS in COVID-19 and ARDS. A "herb-ingredient-target-pathway" network was established using Cytoscape 3.2.7. Results showed that the duration of the negative conversion time of nucleic acid was shorter in patients who received HJSS combination therapy. HJSS combination therapy also relieved fever in patients with severe COVID-19. Network pharmacology analysis identified interleukin (IL) 6, tumor necrosis factor (TNF), vascular endothelial growth factor A (VEGFA), catalase (CAT), mitogen-activated protein kinase (MAPK) 1, tumor protein p53 (TP53), CC-chemokine ligand (CCL2), MAPK3, prostaglandin-endoperoxide synthase 2 (PTGS2), and IL1B as the key targets of HJSS in COVID-19-related ARDS. KEGG analysis suggested that HJSS improved COVID-19-related ARDS by regulating hypoxia-inducible factor (HIF)-1, NOD-like receptor, TNF, T cell receptor, sphingolipid, PI3K-Akt, toll-like receptor, VEGF, FoxO, and MAPK signaling pathways. In conclusion, HJSS can be used as an adjuvant therapy on severe COVID-19. The therapeutic mechanisms may be involved in inhibiting viral replication, inflammatory response, and oxidative stress and alleviating lung injury. Further studies are required to confirm its clinical efficacies and action mechanisms.
Keywords: acute respiratory distress syndrome; coronavirus disease 2019; he-jie-shen-shi decoction; network pharmacology; retrospective cohort study.
Copyright © 2021 Hu, Wang, Wang, Du, Chen, Li, Fan, Li, Sun, Tu, Lu, Zhou and Cui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer HW declared a past co-authorship with several of the authors NL, LW, and HC to the handling editor.
Figures


Similar articles
-
[Mechanism of Xuebijing Injection in treatment of sepsis-associated ARDS based on network pharmacology and in vitro experiment].Zhongguo Zhong Yao Za Zhi. 2023 Jun;48(12):3345-3359. doi: 10.19540/j.cnki.cjcmm.20230202.703. Zhongguo Zhong Yao Za Zhi. 2023. PMID: 37382018 Chinese.
-
[Mechanism of Danggui Sini Decoction in treatment of primary dysmenorrhea based on network pharmacology and molecular docking].Zhongguo Zhong Yao Za Zhi. 2021 Feb;46(4):855-864. doi: 10.19540/j.cnki.cjcmm.20201104.401. Zhongguo Zhong Yao Za Zhi. 2021. PMID: 33645090 Chinese.
-
Examining the effector mechanisms of Xuebijing injection on COVID-19 based on network pharmacology.BioData Min. 2020 Oct 16;13:17. doi: 10.1186/s13040-020-00227-6. eCollection 2020. BioData Min. 2020. PMID: 33082858 Free PMC article.
-
Exploration of the mechanism of Zisheng Shenqi decoction against gout arthritis using network pharmacology.Comput Biol Chem. 2021 Feb;90:107358. doi: 10.1016/j.compbiolchem.2020.107358. Epub 2020 Aug 8. Comput Biol Chem. 2021. PMID: 33243703 Review.
-
Proprietary Medicines Containing Bupleurum chinense DC. (Chaihu) for Depression: Network Meta-Analysis and Network Pharmacology Prediction.Front Pharmacol. 2022 Apr 6;13:773537. doi: 10.3389/fphar.2022.773537. eCollection 2022. Front Pharmacol. 2022. PMID: 35462897 Free PMC article.
Cited by
-
Jian-Ti-Kang-Yi decoction alleviates poly(I:C)-induced pneumonia by inhibiting inflammatory response, reducing oxidative stress, and modulating host metabolism.Front Pharmacol. 2022 Sep 6;13:979400. doi: 10.3389/fphar.2022.979400. eCollection 2022. Front Pharmacol. 2022. PMID: 36147321 Free PMC article.
-
p53/NF-kB Balance in SARS-CoV-2 Infection: From OMICs, Genomics and Pharmacogenomics Insights to Tailored Therapeutic Perspectives (COVIDomics).Front Pharmacol. 2022 May 27;13:871583. doi: 10.3389/fphar.2022.871583. eCollection 2022. Front Pharmacol. 2022. PMID: 35721196 Free PMC article. Review.
-
Role of Traditional Chinese Medicine in Treating Severe or Critical COVID-19: A Systematic Review of Randomized Controlled Trials and Observational Studies.Front Pharmacol. 2022 Jul 15;13:926189. doi: 10.3389/fphar.2022.926189. eCollection 2022. Front Pharmacol. 2022. PMID: 35910363 Free PMC article.
-
Efficacy and mechanisms of traditional Chinese medicine for COVID-19: a systematic review.Chin Med. 2022 Feb 28;17(1):30. doi: 10.1186/s13020-022-00587-7. Chin Med. 2022. PMID: 35227280 Free PMC article. Review.
-
Based on network pharmacology and bioinformatics to analyze the mechanism of action of Astragalus membranaceus in the treatment of vitiligo and COVID-19.Sci Rep. 2023 Mar 8;13(1):3884. doi: 10.1038/s41598-023-29207-6. Sci Rep. 2023. PMID: 36890149 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

