Function and Mechanism of Trimetazidine in Myocardial Infarction-Induced Myocardial Energy Metabolism Disorder Through the SIRT1-AMPK Pathway
- PMID: 34220528
- PMCID: PMC8248253
- DOI: 10.3389/fphys.2021.645041
Function and Mechanism of Trimetazidine in Myocardial Infarction-Induced Myocardial Energy Metabolism Disorder Through the SIRT1-AMPK Pathway
Abstract
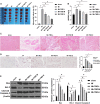
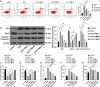
Myocardial energy metabolism (MEM) is an important factor of myocardial injury. Trimetazidine (TMZ) provides protection against myocardial ischemia/reperfusion injury. The current study set out to evaluate the effect and mechanism of TMZ on MEM disorder induced by myocardial infarction (MI). Firstly, a MI mouse model was established by coronary artery ligation, which was then treated with different concentrations of TMZ (5, 10, and 20 mg kg-1 day-1). The results suggested that TMZ reduced the heart/weight ratio in a concentration-dependent manner. TMZ also reduced the levels of Bax and cleaved caspase-3 and promoted Bcl-2 expression. In addition, TMZ augmented adenosine triphosphate (ATP) production and superoxide dismutase (SOD) activity induced by MI and decreased the levels of lipid peroxide (LPO), free fatty acids (FFA), and nitric oxide (NO) in a concentration-dependent manner (all P < 0.05). Furthermore, an H2O2-induced cell injury model was established and treated with different concentrations of TMZ (1, 5, and 10 μM). The results showed that SIRT1 overexpression promoted ATP production and reactive oxygen species (ROS) activity and reduced the levels of LPO, FFA, and NO in H9C2 cardiomyocytes treated with H2O2 and TMZ. Silencing SIRT1 suppressed ATP production and ROS activity and increased the levels of LPO, FFA, and NO (all P < 0.05). TMZ activated the SIRT1-AMPK pathway by increasing SIRT1 expression and AMPK phosphorylation. In conclusion, TMZ inhibited MI-induced myocardial apoptosis and MEM disorder by activating the SIRT1-AMPK pathway.
Keywords: SIRT1-AMPK pathway; apoptosis; myocardial energy metabolism disorder; myocardial infarction; trimetazidine.
Copyright © 2021 Luo, Zhong, Chong, Zhang and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Trimetazidine protects against myocardial ischemia/reperfusion injury by inhibiting excessive autophagy.J Mol Med (Berl). 2018 Aug;96(8):791-806. doi: 10.1007/s00109-018-1664-3. Epub 2018 Jun 29. J Mol Med (Berl). 2018. PMID: 29955901
-
The protective effect of trimetazidine on myocardial ischemia/reperfusion injury through activating AMPK and ERK signaling pathway.Metabolism. 2016 Mar;65(3):122-30. doi: 10.1016/j.metabol.2015.10.022. Epub 2015 Oct 19. Metabolism. 2016. PMID: 26892523 Free PMC article.
-
Trimetazidine prevents macrophage-mediated septic myocardial dysfunction via activation of the histone deacetylase sirtuin 1.Br J Pharmacol. 2016 Feb;173(3):545-61. doi: 10.1111/bph.13386. Epub 2015 Dec 25. Br J Pharmacol. 2016. PMID: 26566260 Free PMC article.
-
Trimetazidine Protects Cardiomyocytes Against Hypoxia/Reoxygenation Injury by Promoting AMP-activated Protein Kinase-dependent Autophagic Flux.J Cardiovasc Pharmacol. 2017 Jun;69(6):389-397. doi: 10.1097/FJC.0000000000000487. J Cardiovasc Pharmacol. 2017. PMID: 28581448
-
Trimetazidine in the Prevention of Tissue Ischemic Conditions.Angiology. 2019 Apr;70(4):291-298. doi: 10.1177/0003319718780551. Epub 2018 Jun 10. Angiology. 2019. PMID: 29888611 Review.
Cited by
-
Efficacy of trimetazidine for myocardial ischemia-reperfusion injury in rat models: a systematic review and meta-analysis.PeerJ. 2025 Jun 6;13:e19515. doi: 10.7717/peerj.19515. eCollection 2025. PeerJ. 2025. PMID: 40492206 Free PMC article.
-
Trend of Galectin-3 Levels in Patients with Non-ST-Elevation and ST-Elevation Myocardial Infarction.Medicina (Kaunas). 2022 Feb 14;58(2):286. doi: 10.3390/medicina58020286. Medicina (Kaunas). 2022. PMID: 35208606 Free PMC article.
-
Circular RNAs modulate cell death in cardiovascular diseases.Cell Death Discov. 2025 May 2;11(1):214. doi: 10.1038/s41420-025-02504-x. Cell Death Discov. 2025. PMID: 40316538 Free PMC article. Review.
-
Deciphering the therapeutic potential of trimetazidine in rheumatoid arthritis via targeting mi-RNA128a, TLR4 signaling pathway, and adenosine-induced FADD-microvesicular shedding: In vivo and in silico study.Front Pharmacol. 2024 Jun 11;15:1406939. doi: 10.3389/fphar.2024.1406939. eCollection 2024. Front Pharmacol. 2024. PMID: 38919260 Free PMC article.
-
Resveratrol activation of SIRT1/MFN2 can improve mitochondria function, alleviating doxorubicin-induced myocardial injury.Cancer Innov. 2023 Mar 30;2(4):253-264. doi: 10.1002/cai2.64. eCollection 2023 Aug. Cancer Innov. 2023. PMID: 38089747 Free PMC article.
References
-
- Chen A., Li W., Chen X., Shen Y., Dai W., Dong Q., et al. (2016). Trimetazidine attenuates pressure overload-induced early cardiac energy dysfunction via regulation of neuropeptide Y system in a rat model of abdominal aortic constriction. BMC Cardiovasc. Disord. 16:225. 10.1186/s12872-016-0399-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

