Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery
- PMID: 34220688
- PMCID: PMC8245059
- DOI: 10.3389/fneur.2021.682151
Fluorescence-Guided Surgery: A Review on Timing and Use in Brain Tumor Surgery
Abstract
Fluorescence-guided surgery (FGS) allows surgeons to have improved visualization of tumor tissue in the operating room, enabling maximal safe resection of malignant brain tumors. Over the past two decades, multiple fluorescent agents have been studied for FGS, including 5-aminolevulinic acid (5-ALA), fluorescein sodium, and indocyanine green (ICG). Both non-targeted and targeted fluorescent agents are currently being used in clinical practice, as well as under investigation, for glioma visualization and resection. While the efficacy of intraoperative fluorescence in studied fluorophores has been well established in the literature, the effect of timing on fluorophore administration in glioma surgery has not been as well depicted. In the past year, recent studies of 5-ALA use have shown that intraoperative fluorescence may persist beyond the previously studied window used in prior multicenter trials. Additionally, the use of fluorophores for different brain tumor types is discussed in detail, including a discussion of choosing the right fluorophore based on tumor etiology. In the following review, the authors will describe the temporal nature of the various fluorophores used in glioma surgery, what remains uncertain in FGS, and provide a guide for using fluorescence as a surgical adjunct in brain tumor surgery.
Keywords: 5-ALA; ICG; extent of resection; fluorescein; fluorescence-guided surgery; timing.
Copyright © 2021 Schupper, Rao, Mohammadi, Baron, Lee, Acerbi and Hadjipanayis.
Conflict of interest statement
CH is a consultant for NX Development Corporation (NXDC) and Synaptive Medical. NXDC, a privately held company, markets Gleolan (5-ALA, aminolevulinic acid hydrochloride). Gleolan is an optical imaging agent approved for the visualization of malignant tissue during glioma surgery. CH is a consultant for NXDC and receives royalty payments for the sale of Gleolan, has also received speaker fees by Carl Zeiss and Leica. FA has received speaker's fees from Carl Zeiss Meditec, Oberkochen, Germany. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
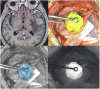
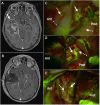
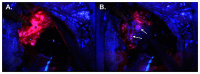
References
-
- Fernandes C, Costa A, Osório L, Lago RC, Linhares P, Carvalho B, et al. . Current Standards of Care in Glioblastoma Therapy. Glioblastoma: Codon Publications. (2017). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

