Staphylococcus epidermidis Has Growth Phase Dependent Affinity for Fibrinogen and Resulting Fibrin Clot Elasticity
- PMID: 34220741
- PMCID: PMC8241941
- DOI: 10.3389/fmicb.2021.649534
Staphylococcus epidermidis Has Growth Phase Dependent Affinity for Fibrinogen and Resulting Fibrin Clot Elasticity
Abstract
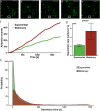
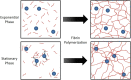
Bacterial infection and thrombosis are highly correlated, especially in patients with indwelling medical devices. Coagulase-negative staphylococci, typified by Staphylococcus epidermidis, are a common cause of medical device infections owing to their biofilm forming capacity which provides protection from antibiotics and host immune response. Attention has been drawn to the interaction between S. epidermidis and host proteins, specifically fibrinogen. However, little is known regarding the impact of the transition from planktonic to biofilm forming phenotype on this interaction. Here we investigate the growth phase dependence of bacteria-fibrinogen interaction and the resulting effect on fibrin clot formation, structure, and mechanics. Flow cytometry demonstrated growth phase dependent affinity for fibrinogen. To mimic intravascular device seeding, we quantified the adhesion of S. epidermidis to a fibrinogen coated surface under continuous flow conditions in vitro. The bacterial deposition rate onto fibrinogen was significantly greater for stationary (5,360 ± 1,776 cells/cm2s) versus exponential phase (2,212 ± 264, cells/cm2 s). Furthermore, the expression of sdrG-a cell surface adhesion protein with specificity for fibrinogen-was upregulated ∼twofold in the stationary versus the exponential phase. Rheometry and confocal microscopy demonstrated that stationary phase S. epidermidis slows clot formation and generates a more heterogeneous fibrin network structure with greater elasticity (G' = 5.7 ± 1.0 Pa) compared to sterile fibrinogen (G' = l.5 ± 0.2 Pa), while exponential phase cells had little effect. This work contributes to the current understanding of the growth phase dependent regulation of bacterial virulence factors and the correlation between bacterial infection and thrombosis.
Keywords: SSPA; SdrG; bacterial adhesion; biofilm; rheometry; thrombosis; virulence.
Copyright © 2021 Vitale, Ma, Sim, Altheim, Martinez-Nieves, Kadiyala, Solomon and VanEpps.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bowden M. G., Heuck A. P., Ponnuraj K., Kolosova E., Choe D., Gurusiddappa S., et al. (2008). Evidence for the “dock, lock, and latch” ligand binding mechanism of the staphylococcal microbial surface component recognizing adhesive matrix molecules (MSCRAMM) SdrG. J. Biol. Chem. 283 638–647. 10.1074/jbc.M706252200 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

