COVID-19 Severity in Obesity: Leptin and Inflammatory Cytokine Interplay in the Link Between High Morbidity and Mortality
- PMID: 34220807
- PMCID: PMC8250137
- DOI: 10.3389/fimmu.2021.649359
COVID-19 Severity in Obesity: Leptin and Inflammatory Cytokine Interplay in the Link Between High Morbidity and Mortality
Abstract
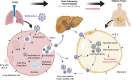
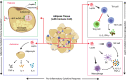
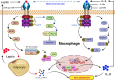
Obesity is one of the foremost risk factors in coronavirus infection resulting in severe illness and mortality as the pandemic progresses. Obesity is a well-known predisposed chronic inflammatory condition. The dynamics of obesity and its impacts on immunity may change the disease severity of pneumonia, especially in acute respiratory distress syndrome, a primary cause of death from SARS-CoV-2 infection. The adipocytes of adipose tissue secret leptin in proportion to individuals' body fat mass. An increase in circulating plasma leptin is a typical characteristic of obesity and correlates with a leptin-resistant state. Leptin is considered a pleiotropic molecule regulating appetite and immunity. In immunity, leptin functions as a cytokine and coordinates the host's innate and adaptive responses by promoting the Th1 type of immune response. Leptin induced the proliferation and functions of antigen-presenting cells, monocytes, and T helper cells, subsequently influencing the pro-inflammatory cytokine secretion by these cells, such as TNF-α, IL-2, or IL-6. Leptin scarcity or resistance is linked with dysregulation of cytokine secretion leading to autoimmune disorders, inflammatory responses, and increased susceptibility towards infectious diseases. Therefore, leptin activity by leptin long-lasting super active antagonist's dysregulation in patients with obesity might contribute to high mortality rates in these patients during SARS-CoV-2 infection. This review systematically discusses the interplay mechanism between leptin and inflammatory cytokines and their contribution to the fatal outcomes in COVID-19 patients with obesity.
Keywords: COVID-19; cytokine; inflammation; leptin; mortality; obesity.
Copyright © 2021 Maurya, Sebastian, Namdeo, Devender and Gertler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ervin RB. Prevalence of Metabolic Syndrome Among Adults 20 Years of Age and Over, by Sex, Age, Race and Ethnicity, and Body Mass Index: United States, 2003-2006. Natl Health Stat Rep (2009) (13):2003–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

