Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated With HIV Infection and Its Treatment
- PMID: 34220817
- PMCID: PMC8250865
- DOI: 10.3389/fimmu.2021.670566
Contribution of Adipose Tissue to the Chronic Immune Activation and Inflammation Associated With HIV Infection and Its Treatment
Abstract
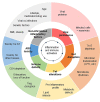
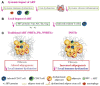
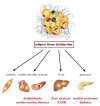
White adipose tissue (AT) contributes significantly to inflammation - especially in the context of obesity. Several of AT's intrinsic features favor its key role in local and systemic inflammation: (i) large distribution throughout the body, (ii) major endocrine activity, and (iii) presence of metabolic and immune cells in close proximity. In obesity, the concomitant pro-inflammatory signals produced by immune cells, adipocytes and adipose stem cells help to drive local inflammation in a vicious circle. Although the secretion of adipokines by AT is a prime contributor to systemic inflammation, the lipotoxicity associated with AT dysfunction might also be involved and could affect distant organs. In HIV-infected patients, the AT is targeted by both HIV infection and antiretroviral therapy (ART). During the primary phase of infection, the virus targets AT directly (by infecting AT CD4 T cells) and indirectly (via viral protein release, inflammatory signals, and gut disruption). The initiation of ART drastically changes the picture: ART reduces viral load, restores (at least partially) the CD4 T cell count, and dampens inflammatory processes on the whole-body level but also within the AT. However, ART induces AT dysfunction and metabolic side effects, which are highly dependent on the individual molecules and the combination used. First generation thymidine reverse transcriptase inhibitors predominantly target mitochondrial DNA and induce oxidative stress and adipocyte death. Protease inhibitors predominantly affect metabolic pathways (affecting adipogenesis and adipocyte homeostasis) resulting in insulin resistance. Recently marketed integrase strand transfer inhibitors induce both adipocyte adipogenesis, hypertrophy and fibrosis. It is challenging to distinguish between the respective effects of viral persistence, persistent immune defects and ART toxicity on the inflammatory profile present in ART-controlled HIV-infected patients. The host metabolic status, the size of the pre-established viral reservoir, the quality of the immune restoration, and the natural ageing with associated comorbidities may mitigate and/or reinforce the contribution of antiretrovirals (ARVs) toxicity to the development of low-grade inflammation in HIV-infected patients. Protecting AT functions appears highly relevant in ART-controlled HIV-infected patients. It requires lifestyle habits improvement in the absence of effective anti-inflammatory treatment. Besides, reducing ART toxicities remains a crucial therapeutic goal.
Keywords: HIV infection; adipose tissue; antiretroviral treatment; chronic immune activation; chronic inflammation; fat.
Copyright © 2021 Bourgeois, Gorwood, Olivo, Le Pelletier, Capeau, Lambotte, Béréziat and Lagathu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection.PLoS Pathog. 2015 Sep 24;11(9):e1005153. doi: 10.1371/journal.ppat.1005153. eCollection 2015 Sep. PLoS Pathog. 2015. PMID: 26402858 Free PMC article.
-
Adipose Tissue in HIV Infection.Compr Physiol. 2017 Sep 12;7(4):1339-1357. doi: 10.1002/cphy.c160028. Compr Physiol. 2017. PMID: 28915327 Free PMC article. Review.
-
Adipose tissue and immune function: a review of evidence relevant to HIV infection.J Infect Dis. 2013 Oct 15;208(8):1194-201. doi: 10.1093/infdis/jit324. Epub 2013 Jul 21. J Infect Dis. 2013. PMID: 23878320 Free PMC article. Review.
-
Adipose tissue as a target of HIV-1 antiretroviral drugs. Potential consequences on metabolic regulations.Curr Pharm Des. 2010 Oct;16(30):3352-60. doi: 10.2174/138161210793563446. Curr Pharm Des. 2010. PMID: 20687886 Review.
-
Adipokines levels in HIV infected patients: lipocalin-2 and fatty acid binding protein-4 as possible markers of HIV and antiretroviral therapy-related adipose tissue inflammation.BMC Infect Dis. 2018 Jan 5;18(1):10. doi: 10.1186/s12879-017-2925-4. BMC Infect Dis. 2018. PMID: 29304747 Free PMC article.
Cited by
-
HIV-Related Immune Activation and Inflammation: Current Understanding and Strategies.J Immunol Res. 2021 Sep 29;2021:7316456. doi: 10.1155/2021/7316456. eCollection 2021. J Immunol Res. 2021. PMID: 34631899 Free PMC article. Review.
-
Distinct Plasma Concentrations of Acyl-CoA-Binding Protein (ACBP) in HIV Progressors and Elite Controllers.Viruses. 2022 Feb 23;14(3):453. doi: 10.3390/v14030453. Viruses. 2022. PMID: 35336860 Free PMC article.
-
Pathophysiology and Clinical Management of Dyslipidemia in People Living with HIV: Sailing through Rough Seas.Life (Basel). 2024 Mar 28;14(4):449. doi: 10.3390/life14040449. Life (Basel). 2024. PMID: 38672720 Free PMC article. Review.
-
Exploring the Pathophysiology of Long COVID: The Central Role of Low-Grade Inflammation and Multisystem Involvement.Int J Mol Sci. 2024 Jun 9;25(12):6389. doi: 10.3390/ijms25126389. Int J Mol Sci. 2024. PMID: 38928096 Free PMC article. Review.
-
Lipodystrophy in HIV: Evolving Challenges and Unresolved Questions.Int J Mol Sci. 2025 Jul 8;26(14):6546. doi: 10.3390/ijms26146546. Int J Mol Sci. 2025. PMID: 40724797 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

