S100a9 Protects Male Lupus-Prone NZBWF1 Mice From Disease Development
- PMID: 34220829
- PMCID: PMC8248531
- DOI: 10.3389/fimmu.2021.681503
S100a9 Protects Male Lupus-Prone NZBWF1 Mice From Disease Development
Abstract
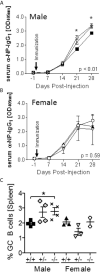
Systemic lupus erythematosus (SLE) is an autoimmune disorder disproportionally affecting women. A similar sex difference exists in the murine New Zealand Black/White hybrid model (NZBWF1) of SLE with all females, but only 30-40% of males, developing disease within the first year of life. Myeloid-derived suppressor cells (MDSCs) are prominent in NZBWF1 males and while depletion of these cells in males, but not females, promotes disease development, the mechanism of suppression remains unknown. S100a9, expressed by neutrophils and MDSCs, has previously been shown to exert immunosuppressive functions in cancer and inflammation. Here we investigated if S100a9 exerts immunosuppressive functions in NZBWF1 male and female mice. S100a9+/+, S100a9+/- and S100a9-/- NZBWF1 mice were followed for disease development for up to 8 months of age. Serum autoantibody levels, splenomegaly, lymphocyte activation, glomerulonephritis and proteinuria were measured longitudinally or at the time of harvest. In accordance with an immunosuppressive function of MDSCs in male mice, S100a9-deficient male NZBWF1 mice developed accelerated autoimmunity as indicated by increased numbers of differentiated effector B and T cells, elevated serum autoantibody levels, increased immune-complex deposition and renal inflammation, and accelerated development of proteinuria. In contrast, female mice showed either no response to S100a9-deficiency or even a slight reduction in disease symptoms. Furthermore, male, but not female, S100a9-/- NZBWF1 mice displayed an elevated type I interferon-induced gene signature, suggesting that S100a9 may dampen a pathogenic type I interferon signal in male mice. Taken together, S100a9 exerts an immunosuppressive function in male NZBWF1 mice effectively moderating lupus-like disease development via inhibition of type I interferon production, lymphocyte activation, autoantibody production and the development of renal disease.
Keywords: MDSC; S100A9; autoantibody; lupus; mouse model; proteinuria; sex specific effects; type I interferon.
Copyright © 2021 Davison, Alberto, Dand, Keller, Patt, Khan, Dvorina, White, Sakurai, Liegl, Vogl and Jorgensen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Programmed Death-Ligand 1 Expression Potentiates the Immune Modulatory Function Of Myeloid-Derived Suppressor Cells in Systemic Lupus Erythematosus.Front Immunol. 2021 Apr 27;12:606024. doi: 10.3389/fimmu.2021.606024. eCollection 2021. Front Immunol. 2021. PMID: 33986739 Free PMC article.
-
Anti-TLR7 Antibody Protects Against Lupus Nephritis in NZBWF1 Mice by Targeting B Cells and Patrolling Monocytes.Front Immunol. 2021 Nov 11;12:777197. doi: 10.3389/fimmu.2021.777197. eCollection 2021. Front Immunol. 2021. PMID: 34868046 Free PMC article.
-
Spontaneous CD4+ T Cell Activation and Differentiation in Lupus-Prone B6.Nba2 Mice Is IFNAR-Independent.Int J Mol Sci. 2022 Jan 14;23(2):874. doi: 10.3390/ijms23020874. Int J Mol Sci. 2022. PMID: 35055071 Free PMC article.
-
Myeloid-derived suppressor cells: Important communicators in systemic lupus erythematosus pathogenesis and its potential therapeutic significance.Hum Immunol. 2021 Oct;82(10):782-790. doi: 10.1016/j.humimm.2021.06.008. Epub 2021 Jul 14. Hum Immunol. 2021. PMID: 34272089 Review.
-
Understanding mechanisms of hypertension in systemic lupus erythematosus.Ther Adv Cardiovasc Dis. 2016 Mar 15;11(1):20-32. doi: 10.1177/1753944716637807. Online ahead of print. Ther Adv Cardiovasc Dis. 2016. PMID: 26985016 Free PMC article. Review.
Cited by
-
The roles of toll-like receptor 4, CD33, CD68, CD69, or CD147/EMMPRIN for monocyte activation by the DAMP S100A8/S100A9.Front Immunol. 2023 Mar 28;14:1110185. doi: 10.3389/fimmu.2023.1110185. eCollection 2023. Front Immunol. 2023. PMID: 37056775 Free PMC article.
-
Exploring the role of APRIL in autoimmunity: implications for therapeutic targeting in systemic lupus erythematosus, rheumatoid arthritis, and Sjögren's syndrome.Front Immunol. 2025 Aug 1;16:1523392. doi: 10.3389/fimmu.2025.1523392. eCollection 2025. Front Immunol. 2025. PMID: 40821822 Free PMC article. Review.
-
SexAnnoDB, a knowledgebase of sex-specific regulations from multi-omics data of human cancers.Biol Sex Differ. 2024 Aug 22;15(1):64. doi: 10.1186/s13293-024-00638-8. Biol Sex Differ. 2024. PMID: 39175079 Free PMC article.
-
The ion transporter Na+-K+-ATPase enables pathological B cell survival in the kidney microenvironment of lupus nephritis.Sci Adv. 2023 Feb 3;9(5):eadf8156. doi: 10.1126/sciadv.adf8156. Epub 2023 Feb 1. Sci Adv. 2023. PMID: 36724234 Free PMC article.
-
Unraveling the Complexities of Myeloid-Derived Suppressor Cells in Inflammatory Bowel Disease.Int J Mol Sci. 2025 Apr 2;26(7):3291. doi: 10.3390/ijms26073291. Int J Mol Sci. 2025. PMID: 40244120 Free PMC article. Review.
References
-
- Kotzin BL, West SG. Systemic Lupus Erythrematosus. In: Rich R, Shearer WT, Kotzin BL, Schroeder JHW, editors. Clinical Immunology. Principles and Practice, 2nd edn. London, UK: Mosby International Limited; (2001). pp. 60.61–60.24.
-
- Lahita RG, Cheng CY, Monder C, Bardin CW. Experience With 19-Nortestosterone in the Therapy of Systemic Lupus Erythematosus: Worsened Disease After Treatment With 19-Nortestosterone in Men and Lack of Improvement in Women. J Rheumatol (1992) 19:547–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

