Short- and Long-Lived Autoantibody-Secreting Cells in Autoimmune Neurological Disorders
- PMID: 34220839
- PMCID: PMC8248361
- DOI: 10.3389/fimmu.2021.686466
Short- and Long-Lived Autoantibody-Secreting Cells in Autoimmune Neurological Disorders
Abstract
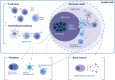
As B cells differentiate into antibody-secreting cells (ASCs), short-lived plasmablasts (SLPBs) are produced by a primary extrafollicular response, followed by the generation of memory B cells and long-lived plasma cells (LLPCs) in germinal centers (GCs). Generation of IgG4 antibodies is T helper type 2 (Th2) and IL-4, -13, and -10-driven and can occur parallel to IgE, in response to chronic stimulation by allergens and helminths. Although IgG4 antibodies are non-crosslinking and have limited ability to mobilize complement and cellular cytotoxicity, when self-tolerance is lost, they can disrupt ligand-receptor binding and cause a wide range of autoimmune disorders including neurological autoimmunity. In myasthenia gravis with predominantly IgG4 autoantibodies against muscle-specific kinase (MuSK), it has been observed that one-time CD20+ B cell depletion with rituximab commonly leads to long-term remission and a marked reduction in autoantibody titer, pointing to a short-lived nature of autoantibody-secreting cells. This is also observed in other predominantly IgG4 autoantibody-mediated neurological disorders, such as chronic inflammatory demyelinating polyneuropathy and autoimmune encephalitis with autoantibodies against the Ranvier paranode and juxtaparanode, respectively, and extends beyond neurological autoimmunity as well. Although IgG1 autoantibody-mediated neurological disorders can also respond well to rituximab induction therapy in combination with an autoantibody titer drop, remission tends to be less long-lasting and cases where titers are refractory tend to occur more often than in IgG4 autoimmunity. Moreover, presence of GC-like structures in the thymus of myasthenic patients with predominantly IgG1 autoantibodies against the acetylcholine receptor and in ovarian teratomas of autoimmune encephalitis patients with predominantly IgG1 autoantibodies against the N-methyl-d-aspartate receptor (NMDAR) confers increased the ability to generate LLPCs. Here, we review available information on the short-and long-lived nature of ASCs in IgG1 and IgG4 autoantibody-mediated neurological disorders and highlight common mechanisms as well as differences, all of which can inform therapeutic strategies and personalized medical approaches.
Keywords: IgG4; autoantibody-mediated disorders; long-lived plasma cells; neurological autoimmunity; rituximab; short-lived.
Copyright © 2021 Zografou, Vakrakou and Stathopoulos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Long-Lasting Rituximab-Induced Reduction of Specific-But Not Total-IgG4 in MuSK-Positive Myasthenia Gravis.Front Immunol. 2020 May 5;11:613. doi: 10.3389/fimmu.2020.00613. eCollection 2020. Front Immunol. 2020. PMID: 32431692 Free PMC article.
-
Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis.JCI Insight. 2017 Sep 7;2(17):e94263. doi: 10.1172/jci.insight.94263. eCollection 2017 Sep 7. JCI Insight. 2017. PMID: 28878127 Free PMC article.
-
Autoimmune Pathology in Myasthenia Gravis Disease Subtypes Is Governed by Divergent Mechanisms of Immunopathology.Front Immunol. 2020 May 27;11:776. doi: 10.3389/fimmu.2020.00776. eCollection 2020. Front Immunol. 2020. PMID: 32547535 Free PMC article. Review.
-
Relation of HLA-DRB1 to IgG4 autoantibody and cytokine production in muscle-specific tyrosine kinase myasthenia gravis (MuSK-MG).Clin Exp Immunol. 2019 Aug;197(2):214-221. doi: 10.1111/cei.13302. Epub 2019 Apr 12. Clin Exp Immunol. 2019. PMID: 30929252 Free PMC article.
-
IgG4-mediated autoimmune diseases: a niche of antibody-mediated disorders.Ann N Y Acad Sci. 2018 Feb;1413(1):92-103. doi: 10.1111/nyas.13561. Epub 2018 Jan 28. Ann N Y Acad Sci. 2018. PMID: 29377160 Free PMC article. Review.
Cited by
-
Anti-pan-neurofascin antibodies induce subclass-related complement activation and nodo-paranodal damage.Brain. 2023 May 2;146(5):1932-1949. doi: 10.1093/brain/awac418. Brain. 2023. PMID: 36346134 Free PMC article.
-
ACHR-Positive Generalized Myasthenia Gravis: The Old is Gold and the New Is for us to Explore.Ann Indian Acad Neurol. 2023 Jul-Aug;26(4):366-367. doi: 10.4103/aian.aian_430_23. Epub 2023 Sep 11. Ann Indian Acad Neurol. 2023. PMID: 37970281 Free PMC article. No abstract available.
-
IgG4 Autoantibodies in Organ-Specific Autoimmunopathies: Reviewing Class Switching, Antibody-Producing Cells, and Specific Immunotherapies.Front Immunol. 2022 Mar 24;13:834342. doi: 10.3389/fimmu.2022.834342. eCollection 2022. Front Immunol. 2022. PMID: 35401530 Free PMC article. Review.
-
Different Patterns of Autoantibody Secretion by Peripheral Blood Mononuclear Cells in Autoimmune Nodopathies.Neurol Neuroimmunol Neuroinflamm. 2024 Sep;11(5):e200295. doi: 10.1212/NXI.0000000000200295. Epub 2024 Aug 22. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 39173087 Free PMC article.
-
Treating myasthenia gravis beyond the eye clinic.Eye (Lond). 2024 Aug;38(12):2422-2436. doi: 10.1038/s41433-024-03133-x. Epub 2024 May 24. Eye (Lond). 2024. PMID: 38789789 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

