Declining Levels of Neutralizing Antibodies Against SARS-CoV-2 in Convalescent COVID-19 Patients One Year Post Symptom Onset
- PMID: 34220870
- PMCID: PMC8242354
- DOI: 10.3389/fimmu.2021.708523
Declining Levels of Neutralizing Antibodies Against SARS-CoV-2 in Convalescent COVID-19 Patients One Year Post Symptom Onset
Abstract
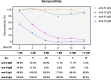
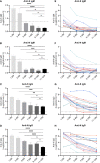
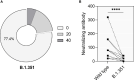
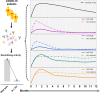
Major advances have been made in understanding the dynamics of humoral immunity briefly after the acute coronavirus disease 2019 (COVID-19). However, knowledge concerning long-term kinetics of antibody responses in convalescent patients is limited. During a one-year period post symptom onset, we longitudinally collected 162 samples from 76 patients and quantified IgM and IgG antibodies recognizing the nucleocapsid (N) protein or the receptor binding domain (RBD) of the spike protein (S). After one year, approximately 90% of recovered patients still had detectable SARS-CoV-2-specific IgG antibodies recognizing N and RBD-S. Intriguingly, neutralizing activity was only detectable in ~43% of patients. When neutralization tests against the E484K-mutated variant of concern (VOC) B.1.351 (initially identified in South Africa) were performed among patients who neutralize the original virus, the capacity to neutralize was even further diminished to 22.6% of donors. Despite declining N- and S-specific IgG titers, a considerable fraction of recovered patients had detectable neutralizing activity one year after infection. However, neutralizing capacities, in particular against an E484K-mutated VOC were only detectable in a minority of patients one year after symptomatic COVID-19. Our findings shed light on the kinetics of long-term immune responses after natural SARS-CoV-2 infection and argue for vaccinations of individuals who experienced a natural infection to protect against emerging VOC.
Keywords: COVID-19; SARS-CoV-2; antibody responses; humoral immunity; spike.
Copyright © 2021 Xiang, Liang, Fang, Lu, Li, Wang, Li, Yang, Shen, Zhu, Wang, Wu, Liu, Lu, Yang, Dittmer, Trilling, Deng and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, et al. Characterization of the Receptor-Binding Domain (RBD) of 2019 Novel Coronavirus: Implication for Development of RBD Protein as a Viral Attachment Inhibitor and Vaccine. Cell Mol Immunol (2020) 17(6):613–20. 10.1038/s41423-020-0400-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

