An Efficient Brome mosaic virus-Based Gene Silencing Protocol for Hexaploid Wheat (Triticum aestivum L.)
- PMID: 34220905
- PMCID: PMC8253535
- DOI: 10.3389/fpls.2021.685187
An Efficient Brome mosaic virus-Based Gene Silencing Protocol for Hexaploid Wheat (Triticum aestivum L.)
Abstract
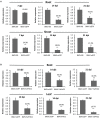
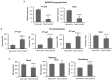
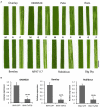
Virus-induced gene silencing (VIGS) is a rapid and powerful method to evaluate gene function, especially for species like hexaploid wheat that have large, redundant genomes and are difficult and time-consuming to transform. The Brome mosaic virus (BMV)-based VIGS vector is widely used in monocotyledonous species but not wheat. Here we report the establishment of a simple and effective VIGS procedure in bread wheat using BMVCP5, the most recently improved BMV silencing vector, and wheat genes PHYTOENE DESATURASE (TaPDS) and PHOSPHATE2 (TaPHO2) as targets. Time-course experiments revealed that smaller inserts (~100 nucleotides, nt) were more stable in BMVCP5 and conferred higher silencing efficiency and longer silencing duration, compared with larger inserts. When using a 100-nt insert and a novel coleoptile inoculation method, BMVCP5 induced extensive silencing of TaPDS transcript and a visible bleaching phenotype in the 2nd to 5th systemically-infected leaves from nine to at least 28 days post inoculation (dpi). For TaPHO2, the ability of BMVCP5 to simultaneously silence all three homoeologs was demonstrated. To investigate the feasibility of BMV VIGS in wheat roots, ectopically expressed enhanced GREEN FLUORESCENT PROTEIN (eGFP) in a transgenic wheat line was targeted for silencing. Silencing of eGFP fluorescence was observed in both the maturation and elongation zones of roots. BMVCP5 mediated significant silencing of eGFP and TaPHO2 mRNA expression in roots at 14 and 21 dpi, and TaPHO2 silencing led to the doubling of inorganic phosphate concentration in the 2nd through 4th systemic leaves. All 54 wheat cultivars screened were susceptible to BMV infection. BMVCP5-mediated TaPDS silencing resulted in the expected bleaching phenotype in all eight cultivars examined, and decreased TaPDS transcript was detected in all three cultivars examined. This BMVCP5 VIGS technology may serve as a rapid and effective functional genomics tool for high-throughput gene function studies in aerial and root tissues and in many wheat cultivars.
Keywords: BMV-VIGS; Brome mosaic virus; PHOSPHATE2; PHYTOENE DESATURASE; Wheat (Triticum aestivum); functional genomics; insert stability; virus-induced gene silencing.
Copyright © 2021 Wang, Chai, Khatabi, Scheible, Udvardi, Saha, Kang and Nelson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

