Development of otology specific outcome measure: Ear Outcome Survey-16 (EOS-16)
- PMID: 34220984
- PMCID: PMC8241709
- DOI: 10.1016/j.joto.2021.01.003
Development of otology specific outcome measure: Ear Outcome Survey-16 (EOS-16)
Abstract
Purpose: An important outcome measure of patient care is the impact on the patient's health-related quality of life (HRQoL). Current ear-specific HRQoL instruments are designed for one diagnosis and emphasize different subdivisions such as symptoms, hearing problems, psychosocial impact, and the need for care. The optimal length of the recall period has not been studied. For these reasons, a new survey is needed that would cover most chronic ear diseases.
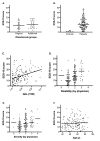
Methods: A preliminary 24-item survey (EOS-24) was created. Untreated adult patients (included n = 186) with one of seven different chronic otologic conditions from all university hospitals in Finland were recruited to respond to EOS-24 and the 15D general HRQoL instrument. The recruiting otologists evaluated the severity of the disease and the disability caused by it. A control group was recruited. Based on the patients' responses in different diagnosis groups, the items were reduced according to pre-defined criteria. The resulting survey was validated using a thorough statistical analysis.
Results: The relevance and necessity of the original 24 items were thoroughly investigated, leading to the exclusion of 8 items and the modification of 1. The remaining 16 items were well-balanced between subdivisions and were useful in all seven diagnosis groups, thus constituting the final instrument, EOS-16. The most suitable recall period was three months.
Conclusions: EOS-16 has been created according to the HRQoL survey guidelines with a versatile nationwide patient population. The survey has been validated and can be used for a wide range of chronic ear diseases as a HRQoL instrument.
Keywords: Chronic ear diseases; HRQoL; Health-related quality of life; Otology; PROM.
© 2021 PLA General Hospital Department of Otolaryngology Head and Neck Surgery. Production and hosting by Elsevier (Singapore) Pte Ltd.
Conflict of interest statement
None. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Figures


References
-
- Aaronson Neil K. Quality of life assessment in clinical trials: methodologic issues. Contr. Clin. Trials. 1989;10:195–208. - PubMed
-
- Bachinger D., Roosli C., Ditzen B. ’Development and validation of the Zurich chronic middle ear inventory (ZCMEI-21): an electronic questionnaire for assessing quality of life in patients with chronic otitis media. Eur. Arch. Oto-Rhino-Laryngol. 2016;273:3073–3081. - PubMed
-
- Baumann I., Kurpiers B., Plinkert P. HNO Springer-Verlag; 2009. Entwicklung und validierung des chronic otitis Media Outcome Test 15 (COMOT-15) - PubMed
-
- Baumhauer Judith F. ’Patient-reported outcomes — are they living up to their potential? N. Engl. J. Med. 2017;377:6–9. - PubMed
LinkOut - more resources
Full Text Sources

