3D Electrospun Nanofiber-Based Scaffolds: From Preparations and Properties to Tissue Regeneration Applications
- PMID: 34221024
- PMCID: PMC8225450
- DOI: 10.1155/2021/8790143
3D Electrospun Nanofiber-Based Scaffolds: From Preparations and Properties to Tissue Regeneration Applications
Abstract
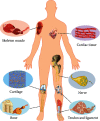
Electrospun nanofibers have been frequently used for tissue engineering due to their morphological similarities with the extracellular matrix (ECM) and tunable chemical and physical properties for regulating cell behaviors and functions. However, most of the existing electrospun nanofibers have a closely packed two-dimensional (2D) membrane with the intrinsic shortcomings of limited cellular infiltration, restricted nutrition diffusion, and unsatisfied thickness. Three-dimensional (3D) electrospun nanofiber-based scaffolds can provide stem cells with 3D microenvironments and biomimetic fibrous structures. Thus, they have been demonstrated to be good candidates for in vivo repair of different tissues. This review summarizes the recent developments in 3D electrospun nanofiber-based scaffolds (ENF-S) for tissue engineering. Three types of 3D ENF-S fabricated using different approaches classified into electrospun nanofiber 3D scaffolds, electrospun nanofiber/hydrogel composite 3D scaffolds, and electrospun nanofiber/porous matrix composite 3D scaffolds are discussed. New functions for these 3D ENF-S and properties, such as facilitated cell infiltration, 3D fibrous architecture, enhanced mechanical properties, and tunable degradability, meeting the requirements of tissue engineering scaffolds were discovered. The applications of 3D ENF-S in cartilage, bone, tendon, ligament, skeletal muscle, nerve, and cardiac tissue regeneration are then presented with a discussion of current challenges and future directions. Finally, we give summaries and future perspectives of 3D ENF-S in tissue engineering and clinical transformation.
Copyright © 2021 Shanshan Han et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Guimarães C. F., Gasperini L., Marques A. P., Reis R. L. The stiffness of living tissues and its implications for tissue engineering. Nature Reviews Materials. 2020;5(5):351–370. doi: 10.1038/s41578-019-0169-1. - DOI
Publication types
LinkOut - more resources
Full Text Sources

