MyrliMax® and Low Back Pain: A Multicentric, Observational, Post-Marketing Surveillance Study in Indian Patients Suffering from Chronic Low Back Pain of Various Pain Intensity
- PMID: 34221157
- PMCID: PMC8224715
- DOI: 10.26574/maedica.2020.16.1.54
MyrliMax® and Low Back Pain: A Multicentric, Observational, Post-Marketing Surveillance Study in Indian Patients Suffering from Chronic Low Back Pain of Various Pain Intensity
Abstract
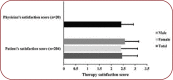
Background: Chronic low back pain (LBP) is the most common musculoskeletal condition affecting a person's quality of life. Over the past decades, a lot of work was done in an attempt to reduce the negative impact of LBP, and help patients recover and maintain a better quality of life. Nevertheless, there is still a lot to be done to fully understand the problem of underlying chronic LBP and a wide gap that exist between basic science and applied rehabilitation research on LBP. Objectives: This was an open label, multicentric, observational, post-marketing surveillance study in a real-world setup designed to evaluate the efficacy and safety of MyrliMax® capsules containing standardised Commiphora myrrha gum resin extract in Indian subjects with chronic LBP varying in intensity. Materials and methods:This study included 204 subjects diagnosed with chronic LBP at the outpatient department of 20 centres under the supervision of a medical doctor. All subjects took MyrliMax® capsules twice daily for 20 days. Visual Analogue Scale (VAS) pain score, rescue medicine requirement, therapy satisfaction scores and safety parameters were assessed as per the schedule. Outcomes:Treatment with MyrliMax® capsules significantly (p<0.01) and progressively reduced the VAS score throughout treatment. A significant pain reduction was observed from the second visit. The mean VAS score was 6.58, 4.66, 2.99 and 1.88 on Day 0, Day 7, Day 14 and Day 20, respectively. A similar trend was also observed in subgroups based on gender and severity score. The need of rescue analgesics/NSAIDs was significantly reduced from the second week, indicating a potential of MyrliMax® capsules to increase the pain threshold. All physicians and patients were satisfied with the efficacy of MyrliMax® capsules assessed by physician's satisfaction score and patient's satisfaction score. There were no significant serious adverse events due to treatment during the study, which indicated that the treatment with MyrliMax® was well tolerated by subjects. Conclusion:MyrliMax® capsule is a potentially effective and safe therapy for pain reduction in patients suffering from chronic LBP. MyrliMax® capsules can be used to reduce pain in NSAIDs intolerant subjects suffering from chronic LBP.
Figures




References
-
- Houze B, El-Khatib H, Arbour C. Efficacy, tolerability, and safety of non-pharmacological therapies for chronic pain: An umbrella review on various CAM approaches. Progr Neuropsychopharmacol Biol Psychiatry. 2017;79(Pt B):192–205. - PubMed
-
- Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;10137:2356–2367. - PubMed
-
- Petzke F, Klose P, Welsch P, et al. Opioids for chronic low back pain: An updated systematic review and meta-analysis of efficacy, tolerability and safety in randomized placebo-controlled studies of at least 4 weeks of double-blind duration. Eur J Pain. 2020;3:497–517. - PubMed
-
- Smith E, Hoy DG, Cross M, Vos T, et al. The global burden of other musculoskeletal disorders: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis. 2014;8:1462–1469. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
