Recent advances in activity-based probes (ABPs) and affinity-based probes (A f BPs) for profiling of enzymes
- PMID: 34221311
- PMCID: PMC8221178
- DOI: 10.1039/d1sc01359a
Recent advances in activity-based probes (ABPs) and affinity-based probes (A f BPs) for profiling of enzymes
Abstract
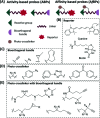
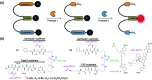
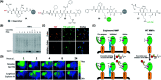
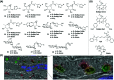
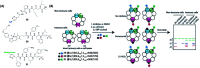
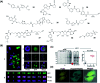
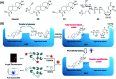
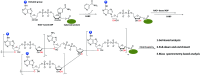
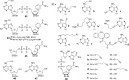
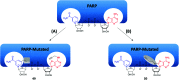
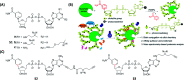
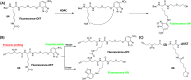
Activity-based protein profiling (ABPP) is a technique that uses highly selective active-site targeted chemical probes to label and monitor the state of proteins. ABPP integrates the strengths of both chemical and biological disciplines. By utilizing chemically synthesized or modified bioactive molecules, ABPP is able to reveal complex physiological and pathological enzyme-substrate interactions at molecular and cellular levels. It is also able to provide critical information of the catalytic activity changes of enzymes, annotate new functions of enzymes, discover new substrates of enzymes, and allow real-time monitoring of the cellular location of enzymes. Based on the mechanism of probe-enzyme interaction, two types of probes that have been used in ABPP are activity-based probes (ABPs) and affinity-based probes (AfBPs). This review highlights the recent advances in the use of ABPs and AfBPs, and summarizes their design strategies (based on inhibitors and substrates) and detection approaches.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
[Advances in applications of activity-based chemical probes in the characterization of amino acid reactivities].Se Pu. 2023 Jan;41(1):14-23. doi: 10.3724/SP.J.1123.2022.05013. Se Pu. 2023. PMID: 36633073 Free PMC article. Review. Chinese.
-
Insights of affinity-based probes for target identification in drug discovery.Eur J Med Chem. 2025 Sep 5;293:117711. doi: 10.1016/j.ejmech.2025.117711. Epub 2025 May 6. Eur J Med Chem. 2025. PMID: 40359656 Review.
-
Current developments in activity-based protein profiling.Bioconjug Chem. 2014 Jul 16;25(7):1181-91. doi: 10.1021/bc500208y. Epub 2014 Jul 2. Bioconjug Chem. 2014. PMID: 24946272 Review.
-
Activity-based protein profiling: Recent advances in medicinal chemistry.Eur J Med Chem. 2020 Apr 1;191:112151. doi: 10.1016/j.ejmech.2020.112151. Epub 2020 Feb 16. Eur J Med Chem. 2020. PMID: 32109778 Review.
-
Advanced Activity-Based Protein Profiling Application Strategies for Drug Development.Front Pharmacol. 2018 Apr 9;9:353. doi: 10.3389/fphar.2018.00353. eCollection 2018. Front Pharmacol. 2018. PMID: 29686618 Free PMC article. Review.
Cited by
-
Mapping the Evolution of Activity-Based Protein Profiling: A Bibliometric Review.Adv Pharm Bull. 2023 Nov;13(4):639-645. doi: 10.34172/apb.2023.082. Epub 2023 May 20. Adv Pharm Bull. 2023. PMID: 38022804 Free PMC article.
-
Target Identification in Anti-Tuberculosis Drug Discovery.Int J Mol Sci. 2023 Jun 22;24(13):10482. doi: 10.3390/ijms241310482. Int J Mol Sci. 2023. PMID: 37445660 Free PMC article. Review.
-
Discovering microbiota functions via chemical probe incorporation for targeted sequencing.Curr Opin Chem Biol. 2025 Feb;84:102551. doi: 10.1016/j.cbpa.2024.102551. Epub 2024 Nov 30. Curr Opin Chem Biol. 2025. PMID: 39615426 Review.
-
Activity-based protein profiling reveals dynamic substrate-specific cellulase secretion by saprotrophic basidiomycetes.Biotechnol Biofuels Bioprod. 2022 Jan 17;15(1):6. doi: 10.1186/s13068-022-02107-z. Biotechnol Biofuels Bioprod. 2022. PMID: 35418096 Free PMC article.
-
Editorial: Mapping enzyme activity: from novel diagnostics to target-based therapeutics, how activity-based probes are improving our understanding of biological catalysts.Front Pharmacol. 2023 Aug 22;14:1271247. doi: 10.3389/fphar.2023.1271247. eCollection 2023. Front Pharmacol. 2023. PMID: 37675044 Free PMC article. No abstract available.
References
-
- Markel U. Essani K. D. Besirlioglu V. Schiffels J. Streit W. R. Schwaneberg U. Chem. Soc. Rev. 2020;49:233–262. - PubMed
-
- Wijdeven R. H. Neefjes J. Ovaa H. Trends Cell Biol. 2014;24:751–760. - PubMed
-
- Uttamchandani M. Lu C. H. Yao S. Q. Acc. Chem. Res. 2009;42:1183–1192. - PubMed
-
- Evans M. J. Cravatt B. F. Chem. Rev. 2006;106:3279–3301. - PubMed
-
- Sanman L. E. Bogyo M. Annu. Rev. Biochem. 2014;83:249–273. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

