Real-world safety and effectiveness of sucroferric oxyhydroxide for treatment of hyperphosphataemia in dialysis patients: a prospective observational study
- PMID: 34221384
- PMCID: PMC8243278
- DOI: 10.1093/ckj/sfaa211
Real-world safety and effectiveness of sucroferric oxyhydroxide for treatment of hyperphosphataemia in dialysis patients: a prospective observational study
Abstract
Background: The iron-based phosphate binder (PB), sucroferric oxyhydroxide (SFOH), is indicated to control serum phosphorus levels in patients with chronic kidney disease on dialysis.
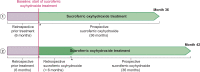
Methods: This non-interventional, prospective, multicentre, cohort study conducted in seven European countries evaluated the safety and effectiveness of SFOH in dialysis patients with hyperphosphataemia in routine practice. Safety outcomes included adverse drug reactions (ADRs) and changes in iron-related parameters. SFOH effectiveness was evaluated by changes-from-baseline (BL) in serum phosphorus and percentage of patients achieving in-target phosphorus levels.
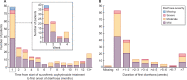
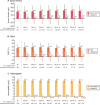
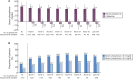
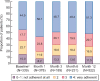
Results: The safety analysis set included 1365 patients (mean observation: 420.3 ± 239.3 days). Overall, 682 (50.0%) patients discontinued the study. Mean SFOH dose during the observation period was 1172.7 ± 539.9 mg (2.3 pills/day). Overall, 617 (45.2%) patients received concomitant PB(s) during SFOH treatment. ADRs and serious ADRs were observed for 531 (38.9%) and 26 (1.9%) patients. Most frequent ADRs were diarrhoea (194 patients, 14.2%) and discoloured faeces (128 patients, 9.4%). Diarrhoea generally occurred early during SFOH treatment and was mostly mild and transient. Small increases from BL in serum ferritin were observed (ranging from +12 to +75 µg/L). SFOH treatment was associated with serum phosphorus reductions (6.3 ± 1.6 mg/dL at BL versus 5.3 ± 1.8 mg/dL at Month 30; ΔBL: -1.0 mg/dL, P < 0.01). Percentage of patients achieving serum phosphorus ≤4.5 mg/dL increased from 12.0% at BL to 34.8% at Month 30, while the percentage achieving serum phosphorus ≤5.5 mg/dL increased from 29.9% to 63.0%.
Conclusions: SFOH has a favourable safety and tolerability profile in a real-world setting, consistent with results of the Phase 3 study. Moreover, SFOH improved serum phosphorus control with a low daily pill burden.
Keywords: chronic kidney disease; end-stage kidney disease; haemodialysis; peritoneal dialysis; phosphate binder.
© The Author(s) 2021. Published by Oxford University Press on behalf of ERA-EDTA.
Figures
Similar articles
-
Sucroferric oxyhydroxide for hyperphosphatemia: a review of real-world evidence.J Nephrol. 2022 Apr;35(3):875-888. doi: 10.1007/s40620-021-01241-5. Epub 2022 Feb 9. J Nephrol. 2022. PMID: 35138627 Free PMC article. Review.
-
The real-world effectiveness of sucroferric oxyhydroxide in European hemodialysis patients: a 1-year retrospective database analysis.BMC Nephrol. 2020 Dec 7;21(1):530. doi: 10.1186/s12882-020-02188-8. BMC Nephrol. 2020. PMID: 33287733 Free PMC article.
-
Safety and effectiveness of sucroferric oxyhydroxide in Spanish patients on dialysis: sub-analysis of the VERIFIE study.Nefrologia (Engl Ed). 2022 Sep-Oct;42(5):594-606. doi: 10.1016/j.nefroe.2021.04.012. Epub 2023 Feb 2. Nefrologia (Engl Ed). 2022. PMID: 36739246
-
Efficacy and Safety of Sucroferric Oxyhydroxide Compared with Sevelamer Carbonate in Chinese Dialysis Patients with Hyperphosphataemia: A Randomised, Open-Label, Multicentre, 12-Week Phase III Study.Nephron. 2024;148(1):22-33. doi: 10.1159/000531869. Epub 2023 Jul 20. Nephron. 2024. PMID: 37473746 Free PMC article. Clinical Trial.
-
Sucroferric oxyhydroxide: a review in hyperphosphataemia in chronic kidney disease patients undergoing dialysis.Drugs. 2015 Apr;75(5):533-42. doi: 10.1007/s40265-015-0366-1. Drugs. 2015. PMID: 25761962 Review.
Cited by
-
Serum Phosphorus Management with Sucroferric Oxyhydroxide as a First-Line Phosphate Binder within the First Year of Hemodialysis.Am J Nephrol. 2024;55(2):127-135. doi: 10.1159/000535754. Epub 2023 Dec 13. Am J Nephrol. 2024. PMID: 38091973 Free PMC article.
-
Sucroferric oxyhydroxide monotherapy for hyperphosphatemia in Indian chronic kidney disease patients undergoing hemodialysis: A phase IV, single-arm, open-label study.World J Nephrol. 2025 Jun 25;14(2):100117. doi: 10.5527/wjn.v14.i2.100117. World J Nephrol. 2025. PMID: 40568335 Free PMC article. Clinical Trial.
-
Sucroferric oxyhydroxide for hyperphosphatemia: a review of real-world evidence.J Nephrol. 2022 Apr;35(3):875-888. doi: 10.1007/s40620-021-01241-5. Epub 2022 Feb 9. J Nephrol. 2022. PMID: 35138627 Free PMC article. Review.
-
Reversal Of Arterial Disease by modulating Magnesium and Phosphate (ROADMAP-study): rationale and design of a randomized controlled trial assessing the effects of magnesium citrate supplementation and phosphate-binding therapy on arterial stiffness in moderate chronic kidney disease.Trials. 2022 Sep 12;23(1):769. doi: 10.1186/s13063-022-06562-9. Trials. 2022. PMID: 36096824 Free PMC article. Clinical Trial.
-
Comparison of the efficacy of sucroferric oxyhydroxide and lanthanum carbonate in the hyperphosphatemia of maintainable Hemodialysis.BMC Pharmacol Toxicol. 2025 Apr 9;26(1):78. doi: 10.1186/s40360-025-00914-2. BMC Pharmacol Toxicol. 2025. PMID: 40205520 Free PMC article. Clinical Trial.
References
-
- Block GA, Hulbert-Shearon TE, Levin NW. et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31: 607–617 - PubMed
-
- Block GA, Klassen PS, Lazarus JM. et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15: 2208–2218 - PubMed
-
- Ganesh SK, Stack AG, Levin NW. et al. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 2001; 12: 2131–2138 - PubMed
-
- Kidney Disease: Improving Global Outcomes CKD-MBD Working Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int 2017; 113 (Suppl): 1–59
LinkOut - more resources
Full Text Sources

