Evaluation of potential drug interactions with sodium zirconium cyclosilicate: a single-center, open-label, one sequence crossover study in healthy adults
- PMID: 34221388
- PMCID: PMC8243284
- DOI: 10.1093/ckj/sfaa222
Evaluation of potential drug interactions with sodium zirconium cyclosilicate: a single-center, open-label, one sequence crossover study in healthy adults
Abstract
Background: Sodium zirconium cyclosilicate (SZC; formerly ZS-9) is an oral potassium binder for the treatment of hyperkalemia in adults. SZC acts in the gastrointestinal tract and additionally binds hydrogen ions in acidic environments like the stomach, potentially transiently increasing gastric pH and leading to drug interactions with pH-sensitive drugs. This study assessed potential pharmacokinetic (PK) interactions between SZC and nine pH-sensitive drugs.
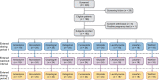
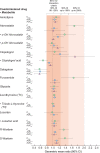
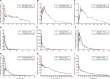
Methods: In this single-dose, open-label, single-sequence cross-over study in healthy adults, amlodipine, atorvastatin, clopidogrel, dabigatran, furosemide, glipizide, levothyroxine, losartan or warfarin were each administered alone and, following a washout interval, with SZC 10 g. Maximum plasma concentration (C max), area under the plasma concentration-time curve from 0 to the last time point (AUC0- t ) and AUC extrapolated to infinity (AUCinf) were evaluated. No interaction was concluded if the 90% confidence interval for the geometric mean ratio (SZC coadministration versus alone) of the PK parameters was within 80-125%.
Results: During SZC coadministration, all PK parameters for amlodipine, glipizide, levothyroxine and losartan showed no interaction, while reductions in clopidogrel and dabigatran C max, AUC0- t and AUCinf (basic drugs) were <50% and increases in atorvastatin, furosemide and warfarin C max (acidic drugs) exceeded the no-interaction range by ˂2-fold.
Conclusions: SZC coadministration was associated with small changes in plasma concentration and exposure of five of the nine drugs evaluated in this study. These PK drug interactions are consistent with transient increases in gastric pH with SZC and are unlikely to be clinically meaningful.
Keywords: drug interactions; gastric pH-sensitive drugs; hyperkalemia; pharmacokinetic analysis; sodium zirconium cyclosilicate.
© The Author(s) 2020. Published by Oxford University Press on behalf of ERA-EDTA.
Figures

Similar articles
-
A Phase 1, open-label, crossover study evaluating the effect of a single dose of sodium zirconium cyclosilicate on the pharmacokinetics of tacrolimus and cyclosporin.Clin Kidney J. 2022 Sep 13;16(1):151-158. doi: 10.1093/ckj/sfac205. eCollection 2023 Jan. Clin Kidney J. 2022. PMID: 36726439 Free PMC article.
-
Phase I Study of the Pharmacodynamics and Safety of Sodium Zirconium Cyclosilicate in Healthy Chinese Adults.Clin Pharmacol Drug Dev. 2022 Mar;11(3):348-357. doi: 10.1002/cpdd.1055. Epub 2022 Jan 8. Clin Pharmacol Drug Dev. 2022. PMID: 34997825 Free PMC article. Clinical Trial.
-
Effect of Concomitant Drugs on Sodium Zirconium Cyclosilicate Hydrate in Artificial Intestinal Juice.Chem Pharm Bull (Tokyo). 2024;72(3):286-293. doi: 10.1248/cpb.c23-00687. Chem Pharm Bull (Tokyo). 2024. PMID: 38447973
-
Effects and Safety of a Novel Oral Potassium-Lowering Drug-Sodium Zirconium Cyclosilicate for the Treatment of Hyperkalemia: a Systematic Review and Meta-Analysis.Cardiovasc Drugs Ther. 2021 Oct;35(5):1057-1066. doi: 10.1007/s10557-020-07134-2. Epub 2021 Jan 18. Cardiovasc Drugs Ther. 2021. PMID: 33459923 Free PMC article.
-
Patiromer and Sodium Zirconium Cyclosilicate in Treatment of Hyperkalemia: A Systematic Review and Meta-Analysis.Curr Ther Res Clin Exp. 2021 Jul 5;95:100635. doi: 10.1016/j.curtheres.2021.100635. eCollection 2021. Curr Ther Res Clin Exp. 2021. PMID: 34367383 Free PMC article. Review.
References
-
- Sarwar CMS, Bhagat AA, Anker SD. et al. Role of hyperkalemia in heart failure and the therapeutic use of potassium binders. Handb Exp Pharmacol 2017; 243: 537–560 - PubMed
-
- US Food and Drug Administration. Kayexalate (sodium polystyrene sulfonate) Powder for Suspension, for Oral or Rectal Use: Prescribing Information. 2017. http://www.accessdata.fda.gov/drugsatfda_docs/label/2017/011287s026lbl.pdf (17 July 2020, date last accessed)
LinkOut - more resources
Full Text Sources

