Scope and heterogeneity of outcomes reported in randomized trials in patients receiving peritoneal dialysis
- PMID: 34221389
- PMCID: PMC8243273
- DOI: 10.1093/ckj/sfaa224
Scope and heterogeneity of outcomes reported in randomized trials in patients receiving peritoneal dialysis
Abstract
Background: Randomized trials can provide evidence to inform decision-making but this may be limited if the outcomes of importance to patients and clinicians are omitted or reported inconsistently. We aimed to assess the scope and heterogeneity of outcomes reported in trials in peritoneal dialysis (PD).
Methods: We searched the Cochrane Kidney and Transplant Specialized Register for randomized trials in PD. We extracted all reported outcome domains and measurements and analyzed their frequency and characteristics.
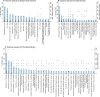
Results: From 128 reports of 120 included trials, 80 different outcome domains were reported. Overall, 39 (49%) domains were surrogate, 23 (29%) patient-reported and 18 (22%) clinical. The five most commonly reported domains were PD-related infection [59 (49%) trials], dialysis solute clearance [51 (42%)], kidney function [45 (38%)], protein metabolism [44 (37%)] and inflammatory markers/oxidative stress [42 (35%)]. Quality of life was reported infrequently (4% of trials). Only 14 (12%) trials included a patient-reported outcome as a primary outcome. The median number of outcome measures (defined as a different measurement, aggregation and metric) was 22 (interquartile range 13-37) per trial. PD-related infection was the most frequently reported clinical outcome as well as the most frequently stated primary outcome. A total of 383 different measures for infection were used, with 66 used more than once.
Conclusions: Trials in PD include important clinical outcomes such as infection, but these are measured and reported inconsistently. Patient-reported outcomes are infrequently reported and nearly half of the domains were surrogate. Standardized outcomes for PD trials are required to improve efficiency and relevance.
Keywords: outcomes; patient-reported outcomes; peritoneal dialysis; trials.
© The Author(s) 2020. Published by Oxford University Press on behalf of ERA-EDTA.
Figures



References
-
- Zazzeroni L, Pasquinelli G, Nanni E. et al. Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: a systematic review and meta-analysis. Kidney Blood Press Res 2017; 42: 717–727 - PubMed
-
- Lozier MR, Sanchez AM, Lee JJ. et al. Comparison of cardiovascular outcomes by dialysis modality: a systematic review and meta-analysis. Perit Dial Int 2019; 39: 306–314 - PubMed
-
- Cho Y, Johnson DW.. Peritoneal dialysis-related peritonitis: towards improving evidence, practices, and outcomes. Am J Kidney Dis 2014; 64: 278–289 - PubMed

