Lurbinectedin in Refractory Diffuse Malignant Peritoneal Mesothelioma: Report of Two Cases
- PMID: 34222029
- PMCID: PMC8249751
- DOI: 10.3389/fonc.2021.704295
Lurbinectedin in Refractory Diffuse Malignant Peritoneal Mesothelioma: Report of Two Cases
Abstract
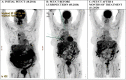
Mesothelioma is a malignancy of serosal membranes. Parietal pleura is the most common site, with peritoneum being the second most frequent location. Malignant peritoneal mesothelioma (MPM) is a rare and aggressive disease. The prognosis is often very poor with median overall survival ranging from 6 to 18 months in patients who are not candidates for cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) due to non-resectable disease or comorbid conditions. For patients with resectable disease, CRS and HIPEC have become the standard of care. However, for patients with unresectable malignant mesothelioma there is unfortunately no effective systemic treatment beyond the first line. Based on the results of a recent phase II trial, lurbinectedin has clinical activity and acceptable toxicity in the second- and third-line treatment of malignant pleural mesothelioma. However, until present, no data have been available for patients with MPM and for patients who become refractory after multiple treatment lines. We report on two patients with metastatic MPM who achieved durable disease control of 10+ and 8 months with lurbinectedin in the fourth and fifth treatment line, respectively.
Keywords: case report; durable response; lurbinectedin; palliative chemotherapy; peritoneal mesothelioma; peritoneal tumor.
Copyright © 2021 Gros, Szturz, Diciolla, Kirchner, Peters, Schaefer, Hubner and Digklia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma: patient selection and special considerations.Cancer Manag Res. 2019 May 7;11:4231-4241. doi: 10.2147/CMAR.S170300. eCollection 2019. Cancer Manag Res. 2019. PMID: 31190990 Free PMC article. Review.
-
Malignant peritoneal mesothelioma: a review.Ann Transl Med. 2017 Jun;5(11):236. doi: 10.21037/atm.2017.03.96. Ann Transl Med. 2017. PMID: 28706904 Free PMC article. Review.
-
Adjuvant dendritic cell based immunotherapy (DCBI) after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal mesothelioma, a phase II single centre open-label clinical trial: rationale and design of the MESOPEC trial.BMJ Open. 2019 May 14;9(5):e026779. doi: 10.1136/bmjopen-2018-026779. BMJ Open. 2019. PMID: 31092657 Free PMC article.
-
Lurbinectedin as second- or third-line palliative therapy in malignant pleural mesothelioma: an international, multi-centre, single-arm, phase II trial (SAKK 17/16).Ann Oncol. 2020 Apr;31(4):495-500. doi: 10.1016/j.annonc.2019.12.009. Epub 2020 Jan 16. Ann Oncol. 2020. PMID: 32085891 Clinical Trial.
-
Malignant peritoneal mesothelioma literature review: past, present, and future.Dig Med Res. 2022 Jun;5:29. doi: 10.21037/dmr-22-19. Epub 2022 Jun 30. Dig Med Res. 2022. PMID: 36061260 Free PMC article.
References
-
- Helm JH, Miura JT, Glenn JA, Marcus RK, Larrieux G, Jayakrishnan TT, et al. . Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma: A Systematic Review and Meta-Analysis. Ann Surg Oncol (2015) 22(5):1686–93. 10.1245/s10434-014-3978-x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

